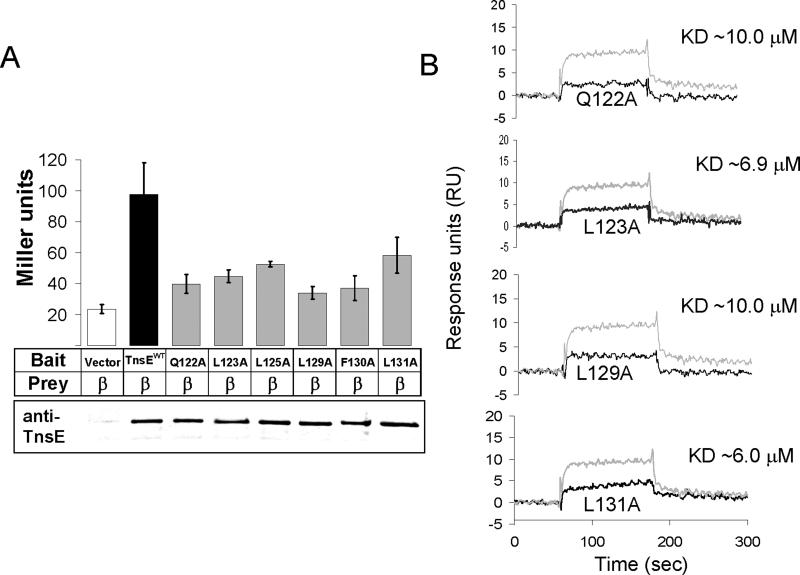
Figure 4.
A. Quantification of the yeast two-hybrid assay reveals a defect in the β interaction with TnsE proteins with alanine substitutions in the putative β interaction motif. The alanine substitutions in the putative β clamp binding motif consistently reduce TnsE-β interaction levels. Interaction was measured by Miller assay and is reported in Miller Units (Miller, 1992). Western blots using anti-TnsE antibodies are given below the bar graph. Error bars indicate standard error of the mean (n=4).
B. Surface Plasmon Resonance results for purified TnsEβMA mutants confirm a loss in interaction between TnsE and β in vitro. All four TnsEβMA mutants tested show a decrease in β interaction. Reference subtracted results from 1 μM of each of the TnsEβMA mutant proteins tested are shown (black lines) overlaid with results from 1 μM TnsEWT (gray lines) for comparison.