Abstract
PURPOSE
After initial surgery, there has been no established consensus regarding adjunctive therapy for patients with uterine carcinosarcoma (CS). This study was designed to compare patient outcome following treatment with adjuvant whole abdominal irradiation (WAI) versus (vs) chemotherapy for patients with this rare group of female pelvic malignancies.
PATIENTS AND METHODS
Eligible, consenting women with stage I-IV uterine CS, no more than 1 cm postsurgical residuum and/or no extra-abdominal spread had their treatments randomly assigned as either WAI or three cycles of cisplatin (C), ifosfamide (I), and mesna (M).
RESULTS
232 patients were enrolled, of whom 206 (WAI=105; CIM=101) were deemed eligible. Patient demographics and characteristics were similar between arms. FIGO stage (both arms) was: I=64 (31%); II=26 (13%); III=92 (45%); IV=24 (12%). The estimated crude probability of recurring within 5 years was 58% (WAI) and 52% (CIM). Adjusting for stage and age, the recurrence rate was 21% lower for CIM patients than for WAI patients, (relative hazard [RH] = 0.789, 95% confidence interval [CI]: (0.530 –1.176), p = 0.245, 2-tail test). The estimated death rate was 29% lower among the CIM group (RH = 0.712, 95% CI: 0.484 – 1.048, p = 0.085, 2-tail test).
CONCLUSION
We did not find a statistically significant advantage in recurrence rate or survival for adjuvant CIM over WAI in patients with uterine CS. However, the observed differences favor the use of combination chemotherapy in future trials.
INTRODUCTION
Carcinosarcoma (CS) of the uterus (formerly referred to as malignant mixed mullerian sarcoma or mixed mesodermal sarcoma) is a rare class of malignant female pelvic neoplasms. According to the American Cancer Society, there will be approximately 41,200 new cases of cancers of the uterus for the year 2006 with roughly 4% or 1,600 being uterine sarcomas (US).1 Historically, carcinosarcomas (CSs) of the uterus have been described as being the most common subtype of US that display both epithelial and stromal differentiation.2 Furthermore, the sarcomatous component of this entity has been previously subdivided into homologous (e.g. leiomyosarcoma, fibrosarcoma, malignant fibrous histiocytoma, or undifferentiated sarcoma) versus heterologous (rhabdomyosarcoma, chondrosarcoma, osteosarcoma, or liposarcoma) cell types. However, there is now considerable evidence that uterine CSs originate from a monoclonal cell.3,4 In fact, the current accepted World Health Organization (WHO) classification for this group of female pelvic malignancies is no longer as uterine sarcomas but rather as mixed epithelial and stromal tumors.5
The primary modality of therapy for uterine CS is surgery, consisting of the following: total abdominal hysterectomy (TAH), bilateral salpingo-oophorectomy (BSO), retroperitoneal lymph node dissection (RPLND), and collection of peritoneal washings along with resection of any gross intra-abdominal disease.6 The most important predictor of prognosis for this group of patients has been reported as being surgical stage of disease at diagnosis.7
A Phase III randomized trial (Protocol 20) conducted by the Gynecologic Oncology Group (GOG) compared postoperative doxorubicin versus no chemotherapy in patients with newly diagnosed Stage I and II US, including CS and leiomyosarcoma.8 There was also the opportunity for study patients to receive optional external beam radiation therapy (EBRT) to the pelvis prior to the randomization to adjuvant treatment or observation. This study did not demonstrate either a progression-free or overall survival advantage for either of these treatment approaches. However, it did report, retrospectively, a significant reduction in pelvic recurrences (23% vs 10%, p=0.028) for patients treated with EBRT.9
The GOG has also conducted a nonrandomized, cohort study (Protocol 40) evaluating the outcome of patients with newly diagnosed clinical stage I or II US (including CS). Of those with CS who had relapses, 63 out of 301 (20.9%) failed with a pelvic component, while only 28 out of 301 (9.3%) recurred first in the lung.10 Based on these data, whole abdominal radiotherapy was incorporated as a study treatment in the current study.
A GOG phase II trial (Protocol 117), which preceded the activation of this present trial, established the initial feasibility of employing combination cisplatin and Ifosfamide along with mesna (CIM) for the adjuvant treatment of stage I and II uterine carcinosarcomas.11 Therefore, this regimen was chosen as the chemotherapeutic arm for the current study.
Thus, this randomized Phase III trial (GOG Protocol 150) was designed to compare whole abdominal irradiation (WAI) versus CIM with respect to overall survival (OS), recurrence rates, and therapeutic toxicities in patients with uterine carcinosarcomas.
At the time of this study’s inception, there was a consensus among the study investigators as well as the GOG Protocol Committee members that a three “arm” study in this rare disease that included an “observation only” cohort was not feasible.
PATIENTS AND METHODS
Patient Eligibility
Previously untreated patients with stages I, II, III, and IV primary carcinosarcomas of the uterus or cervix (without any demonstrable parenchymal hepatic involvement or extra-abdominal distant disease) were eligible for this trial. The stages of uterine CS have been well described2 and may be summarized as follows: stage I is confined to the uterus; stage II involves the corpus and cervix; stage III extends outside the uterus but not outside the true pelvis; and stage IV extends outside the true pelvis or involves bladder or rectum. Eligibility required a patient to have a TAH, BSO, and maximal resection of all gross intra-abdominal/pelvic disease, including macroscopically involved pelvic and para-aortic nodes, leaving no residual disease any larger than 1 cm. Peritoneal cytology and RPLND were optional if there were no intraoperative clinical manifestations of residual disease within the abdomen and pelvis. Adequate hematologic (WBC ≥ 3,000/uL, platelets ≥ 100,000/uL, and granulocytes ≥ 1,500/uL), renal (serum creatinine ≤ 1.5 mg % or creatinine clearance ≥ 50 mL/minute), and hepatic (serum bilirubin ≤ 1.5X the institutional value, serum AST ≤ 3X the institutional value, and serum albumin ≥ 3) functions were required. In addition, eligible patients were required to have a GOG Performance Status of 0, 1, or 2 and a normal chest x-ray (no other imaging studies were required).
Additionally, patients who had a prior history of receiving radiotherapy and/or chemotherapy or who had a concomitant malignancy (other than non-melanoma skin cancer) within five years of being diagnosed with uterine CS were ineligible. However, patients who had received prior hormonal manipulation (not evaluated in this study) were eligible for entry.
Protocol therapy was to be started within eight weeks following initial surgery. The institutional review board (IRB) at each participating institution approved this trial, and all patients provided written informed consent in compliance with all institutional, state, and federal regulations before study entry.
Radiation Therapy
WAI was to be delivered by external beam radiation therapy (EBRT) to the abdomen and pelvis that involved a minimum beam energy of 4 MV photons and utilized an anterior/posterior (AP) and posterior/anterior (PA) summated technique. The field borders for WAI involved 1 cm margins on the diaphragm superiorly, the inguinal ligament inferiorly, and the lateral aspect of the peritoneal margin laterally. At the outset of this protocol, the whole abdomen was treated to a total dose of 30 Gy at 1 Gy per fraction, two fractions per day, and five days each week with a minimum of six hours between morning and afternoon fractions (hyperfractionation). Due to slow patient accrual, in August of 1996, the dose fractionation schedule was changed to once-daily fractions of 1.5 Gy for five days each week to the same total dose to the whole abdomen of 30 Gy.
The true pelvis was treated with a boost requiring a four-field “box” set-up that included not only AP/PA beam portals but also opposed lateral fields. The pelvic field borders included the S1/S2 interspace superiorly, the mid-level portion of the obturator foramen inferiorly, and 1 cm beyond the widest aspect of the true pelvic laterally. At the initiation of this study, the true pelvis as a boost was treated to a total dose of 20 Gy at 1 Gy per fraction, two fractions per day for five days each week with same six hour time interval between fractions as was initially done for the WAI (cumulative true pelvic dose of 50 Gy). As stated above, the fractionation schedule was also changed in August of 1996 for this portion of radiotherapy to once-daily fractions of 1.8 Gy for five days each week to a total dose of 19.8 Gy (cumulative true pelvic dose of 49.8 Gy).
Shaped normal tissue blocking was required for both WAI and true pelvic EBRT to reduce the risk of both acute and chronic radiation damage. Of note is the fact that no shielding of the liver was allowed during WAI; however, both kidneys were required to be shielded in the PA beam beginning with the first day of WAI in order to limit the exposure to the kidneys at no more than 20 Gy total dose.
All fields were to be treated daily, five days per week. If more than a two-week treatment interruption was needed, the study chair was to be notified. Resumption of study radiotherapy was at the treating physician’s discretion, and patient follow-up was required regardless of treatment compliance.
Chemotherapy
Chemotherapy comprised intravenous (IV) cisplatin (20 mg/m2/day × 4 days) that was to be followed by a one hour IV administration of Ifosfamide (1.5 g/m2/day IV × 4 days) with mesna (120 mg/m2 IV bolus over 15 minutes on day one, followed by 1.5 g/m2/day IV continuous infusion × 4 days beginning with day one) every three weeks for three cycles. It was recommended that hydration be maintained by IV administration of 1 L over several hours preferably with either normal or one-half normal saline prior to initiation of chemotherapy in order to maintain urine output of at least 100 mL/hour. IV fluid and electrolyte repletion was permitted as medically indicated during the four-day course of chemotherapy.
Cisplatin administration was required prior to Ifosfamide therapy and was to be reconstituted at a concentration of approximately 1 mg/mL and infused at a rate of 1 mg/minute. Dose modifications for toxicities of cisplatin and Ifosfamide were permitted but not for mesna administration. Patients having chemotherapy delayed for at least six weeks were to be removed from study treatment. The use of growth factors was permitted during study chemotherapy and was not analyzed in this report.
Follow-Up Patient Assessments
Patients were to be evaluated weekly during study therapies by their treating physicians and were required to have monitoring of complete blood counts (CBC), and serum creatinine as well as serum bilirubin and serum AST during the WAI portion of treatment and urinalysis during ifosfamide therapy. In addition, prior to each cycle of chemotherapy, patients in that cohort were required to be examined by their treating physician as well as to have the CBC, serum creatinine, serum bilirubin, serum AST, serum alkaline phosphatase, and serum albumin.
After protocol treatment, patients were evaluated every three months for the first two years and then every six months thereafter by a treating physician with CBC, serum creatinine, serum bilirubin, serum AST, and CA-125 level (required prior to study entry). A chest x-ray was required every six months following completion of study treatment for the first two years and then yearly thereafter. Patients were observed for survival, disease recurrence, and adverse events.
Quality Assurance Reviews
The members of the GOG Radiation Oncology Committee reviewed the simulation and port films, isodose computations, and radiation summary forms. Dosimetry quality control was the purview of the Radiological Physics Center in Houston, Texas. The study chair (AHW) reviewed all patient data forms for completeness. Members of the GOG Gynecologic Oncology Committee reviewed pretreatment op reports and discharge summaries. Pathology materials, including microscope slides documenting primary and metastatic disease, were reviewed by members of the GOG Pathology Committee. Only the centralized Gynecologic Oncology Committee and the Pathology Committee reviews were used to determine an individual’s eligibility and both of these reviews were performed without knowledge of outcome.
Statistical Considerations
A pre-determined sequence of treatment assignments was centrally maintained at the GOG Statistical and Data Center in Buffalo, New York. The list of treatment assignments was created by concatenating randomly selected balanced blocks of permuted treatments. A separate list of treatment assignments was maintained for each GOG member institution and its affiliated clinics. The complete list of treatment assignments remained concealed and only the next unassigned treatment was revealed after each patient was successfully registered onto the study. This report includes an accounting of all patients successfully enrolled onto this study and categorizes them, unless otherwise noted, according to their assigned treatments regardless of compliance.
The primary endpoints in this study for assessing treatment efficacy are death and recurrence rates. An individual’s survival is assessed from the date she was registered onto the study to the date of death from any cause or, for living patients, the date of last contact. The recurrence-free interval was assessed from the date of entry onto the protocol to date when clinically evident disease was observed or the date of last contact for those without any evidence of recurrence. The times at risk of recurrence are censored at the date of last contact for patients who were alive and without any clinical evidence of tumor. The duration of OS and recurrence-free interval are calculated without regard for subsequent anti-cancer treatments. The treatment comparisons included in this report include all patients considered eligible for the study for an intention-to-treat analysis, unless otherwise noted. Heterogeneity of the treatment effects across subgroups of patients was assessed with fixed-effects models.
The targeted accrual for this study was 216 eligible patients and the data was to be considered sufficiently mature for a final analysis when there were at least 91 recurrences and 91 deaths reported. Assuming proportional hazards, this study size would provide at least an 80% chance of rejecting the null hypothesis, if one of these treatment regimen reduces the death rate (or recurrence rate) 45% relative to the other treatment regimen when the type I error for each hypothesis is limited to 5% for a two-tail test. Historical data (Protocol 40) indicated that approximately 50% of these patients would fail within three years of diagnosis. Therefore, a 45% reduction in the recurrence rate would be comparable to reducing the probability of recurring within three years to 32%.
The study design specified that an interim analysis was to be performed once two-thirds of the targeted sample size had been enrolled. The design specified that accrual termination would be considered, if the null hypothesis assessing no treatment effect on the risk or recurrence could be rejected with the critical p-value set to 0.005.
For the purposes of this report, the product-limit method was used to provide non-parametric estimates of the survival time distributions.12 The cumulative probability of disease recurrence, accounting for the competing risk of noncancer-related death, is estimated using cumulative incidence procedures.13 A logrank test stratified by stage (I vs II vs III vs IV) and age (< 65 vs ≥ 65 years old) was employed to assess the hypothesis that the event rates on each treatment are equal. A proportional hazards model was utilized to estimate the relative hazards of either recurrence or death.14 The sites of first-recurrence for each patient were classified as vaginal, pelvic, abdomen, lung, or other distant sites and patients with multiple sites of first recurrence were counted once for each site.
Adverse events of treatment were classified as being either acute or late toxicities. A toxicity that occurred during study therapy was identified as acute, while those that either persisted or developed after completion of treatment were separately identified as late or chronic toxicities. Toxicities which occurred after a patient recurred and started second-line therapies were not counted among the late or chronic effects of treatment. The October 1988 GOG Common Toxicity Criteria were used to grade toxicities.15 For the purpose of this report, only those patients who initiated their study treatment are included in the toxicity summaries.
RESULTS
Study Timeline
This study was opened for enrollment on December 27, 1993. However, by June 1996, only 43 patients had been enrolled. A poll of the GOG membership indicated that the twice-daily radiation scheduled was an impediment to study enrollment. Therefore, the study was amended in August 1996 to alter the EBRT treatment schedule to daily fractionation as described in Methods Section. By June 2001, 154 patients had been enrolled, of whom 142 were considered eligible. Therefore, the scheduled interim analysis was prepared and presented to the Data Monitoring Committee (DMC). The interim analysis included 57 recurrences and the treatment comparison with respect to recurrence rates did not cross the threshold for statistical significance (p>0.005). Following a complete review of the available data, the DMC recommended completing the trial as planned. Ultimately, 232 patients were enrolled onto the study before it was closed in March 2005. The current report is prepared from data frozen on November 1, 2006.
Patient Characteristics
Two hundred thirty-two women were enrolled in this study, of whom 206 were eligible. Twenty-five patients were excluded based on review of the histology as determined by the GOG Pathology Committee’s central review. One other patient was excluded due to inappropriate residual disease as determined by the GOG Gynecologic Oncology Committee review.
The treatment arms were reasonably balanced with respect to patient characteristics (Table 1). The median patient age at initial diagnosis was 68 years for the WAI versus 64.7 years for the CIM group. Sixty-eight percent of the patients were White, 28% were Black and the remaining 3% were Hispanic, Asian, or Pacific Islander, The initial performance status was considered normal for 52% for those randomized to WAI versus 63% or those randomized to CIM. The proportion of all patients with homologous CS was slightly greater (54%) than those with heterologous tumors (46%), and these proportions were reasonably similar within each treatment group. Patients with stage III disease were the most common stage for both treatment cohorts (43% vs 46%). Eleven patients (5%) had gross residual disease (largest diameter ≤ 1 cm) following cytoreductive surgery.
Table 1.
Patient Characteristics
| WAI (n = 105) |
CIM (n = 101) |
|||
|---|---|---|---|---|
| Characteristic | No. | % | No. | % |
| Age at study entry, years | ||||
| ≤ 50 | 4 | 3.8 | 7 | 7.0 |
| 50–59 | 16 | 15.2 | 22 | 21.8 |
| 60–69 | 48 | 45.7 | 41 | 40.6 |
| 70–80 | 35 | 33.3 | 27 | 26.7 |
| > 80 | 2 | 1.9 | 4 | 4.0 |
| Race | ||||
| Black | 27 | 25.7 | 31 | 30.7 |
| Hispanic | 2 | 1.9 | 2 | 2.0 |
| White | 75 | 71.4 | 66 | 65.3 |
| Other | 1 | 1.0 | 2 | 2.0 |
| Performance Status | ||||
| 0 | 55 | 52.4 | 64 | 63.4 |
| 1 | 49 | 46.7 | 36 | 35.6 |
| 2 | 1 | 1.0 | 1 | 1.0 |
| Cell Type | ||||
| Homologous | 59 | 56.2 | 53 | 52.5 |
| Heterologous | 46 | 43.8 | 48 | 47.5 |
| FIGO stage+ | ||||
| I | 35 | 33.3 | 29 | 28.7 |
| II | 11 | 10.5 | 15 | 14.9 |
| III | 45 | 42.9 | 47 | 46.5 |
| IV | 14 | 13.3 | 10 | 9.9 |
| Gross Residual Disease | 6 | 5.7 | 5 | 4.9 |
Abbreviations: WAI, whole abdominal irradiation; CIM, cisplatin, ifosfamide with mesna chemotherapy; No, patient number; FIGO, International Federation of Gynecology and Obstetrics.
Includes Native American, Asian, and Pacific Islander.
Nodal evaluation and collection of peritoneal cytology were optional.
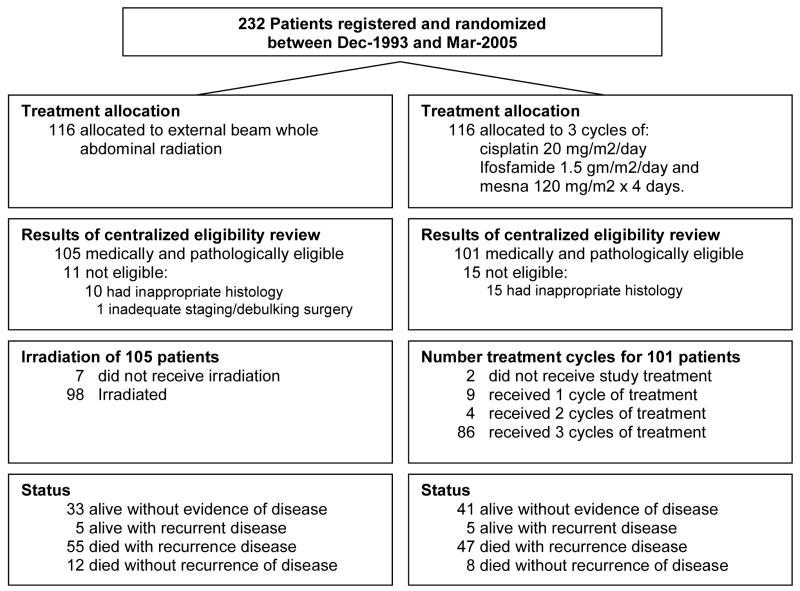
Treatment
Among the 105 eligible patients randomized to WAI, seven did not receive the assigned study treatment (Figure 1). The first, second, and third quartiles of the radiation dose delivered to the abdomen was 30 Gy and the median number of treatment fractions to the abdomen was 20. The median dose delivered during pelvic boost was 49.8 Gy [first quartile (Q1)=49 Gy and the third quartile (Q3)=50 Gy], and the median number of treatment fractions to the pelvis was 11. The median duration of radiation treatment was 43 days (Q1=37 days, Q3=47 days).
Figure 1.
GOG Protocol 150 CONSORT Diagram
Among the 101 eligible patients randomized to CIM, two did not initiate the assigned study treatment. Eighty-six patients (85%) completed all three cycles of chemotherapy. The median time for completing three cycles of therapy was 49 days (Q1=43 days, Q3=56 days). The number of chemotherapy cycles completed is summarized in Figure 1.
Adverse Effects of Treatment
The severity of acute and chronic toxicities are summarized in Tables 2 and 3, respectively for the 197 patients who initiated their study treatment. The nine patients who did not receive any of their study treatment are not included in these summaries.
Table 2.
Patients Experiencing Acute Adverse Events
| WAI Regimen (n = 98)† |
CIM Regimen (n = 99)† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||
| Adverse Event | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Anemia | 88 | 5 | 4 | 1 | 0 | 41 | 12 | 35 | 10 | 1 |
| Gastrointestinal | 33 | 33 | 21 | 8 | 3 | 37 | 33 | 19 | 8 | 2 |
| Genitourinary | 82 | 13 | 3 | 0 | 0 | 77 | 12 | 10 | 0 | 0 |
| Renal | 98 | 0 | 0 | 0 | 0 | 97 | 1 | 1 | 0 | 0 |
| Hepatic | 94 | 2 | 0 | 1 | 1 | 94 | 4 | 1 | 0 | 0 |
| Fever | 97 | 1 | 0 | 0 | 0 | 87 | 3 | 9 | 0 | 0 |
| Infection | 97 | 0 | 0 | 1 | 0 | 98 | 0 | 0 | 0 | 1* |
| Fatigue | 92 | 5 | 0 | 1 | 0 | 77 | 13 | 6 | 2 | 1 |
| Alopecia | 98 | 0 | 0 | 0 | 0 | 54 | 12 | 33 | 0 | 0 |
| Peripheral Neuropathy | 97 | 1 | 0 | 0 | 0 | 87 | 6 | 4 | 2 | 0 |
| Central Neuropathy | 96 | 2 | 0 | 0 | 0 | 79 | 9 | 4 | 7 | 0 |
| Allergy | 98 | 0 | 0 | 0 | 0 | 95 | 2 | 2 | 0 | 0 |
| Cutaneous | 89 | 4 | 5 | 0 | 0 | 96 | 3 | 0 | 0 | 0 |
| Cardiovascular | 97 | 0 | 0 | 0 | 1 | 92 | 2 | 1 | 2 | 2 |
| Pulmonary | 97 | 0 | 1 | 0 | 0 | 93 | 3 | 3 | 0 | 0 |
| Pain | 94 | 2 | 2 | 0 | 0 | 86 | 9 | 3 | 1 | 0 |
| Metabolic | 97 | 1 | 0 | 0 | 0 | 90 | 2 | 2 | 3 | 2 |
Abbreviations: WAI, whole abdominal irradiation; CIM, cisplatin, ifosfamide with mesna chemotherapy.
Adverse events summarized for those who initiated study treatment.
One patient died of a systemic infection complicated by neutropenia which was attributed to CIM treatment.
Table 3.
Patients Experiencing Chronic Adverse Events
| WAI Regimen (n = 98)† |
CIM Regimen (n = 99)† |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Grade | Grade | |||||||||
| Adverse Event | 0 | 1 | 2 | 3 | 4 | 0 | 1 | 2 | 3 | 4 |
| Hematologic | 89 | 3 | 3 | 2 | 1 | 96 | 2 | 1 | 0 | 0 |
| Neurologic | 93 | 2 | 1 | 2 | 0 | 91 | 4 | 3 | 1 | 0 |
| Gastrointestinal | 75 | 13 | 4 | 5 | 1 | 89 | 10 | 0 | 0 | 0 |
| Genitourinary | 89 | 7 | 0 | 2 | 0 | 91 | 4 | 2 | 2 | 0 |
| Lymphedema | 93 | 2 | 2 | 1 | 0 | 97 | 2 | 0 | 0 | 0 |
| Hepatic | 93 | 2 | 0 | 1 | 2* | 99 | 0 | 0 | 0 | 0 |
| Cardiovascular | 96 | 0 | 1 | 1 | 0 | 98 | 0 | 0 | 1 | 0 |
| Cutaneous | 92 | 0 | 6 | 0 | 0 | 99 | 0 | 0 | 0 | 0 |
Abbreviations: WAI, whole abdominal irradiation; CIM, cisplatin, ifosfamide with mesna chemotherapy.
Adverse events summarized for those who initiated study treatment.
Two patient deaths were attributed to radiation induced hepatitis.
The common acute side effects are gastrointestinal, anemia, alopecia, genitourinary, and fatigue. The common late adverse effects are gastrointestinal, genitourinary, neurologic, hematologic, and lymphedema.
When considering grade 3 or 4 acute effects of treatment, anemia (11 vs 1, p < 0.01) and neuropathy [central and peripheral] (9 vs 0, p < 0.01) occurred more often among those treated with CIM than radiotherapy. Regarding grade 2, 3 or 4 late effects, gastrointestinal events occurred more often among those treated with WAI than chemotherapy (10 vs 0, p < 0.001). Moreover, two patients in the radiotherapy cohort died as a direct consequence of radiation hepatitis, while one patient in the chemotherapy arm died of an acute systemic blood infection which originated at the port site and was complicated by neutropenia.
Patterns of Initial Treatment Failure
There have been 112 recurrences reported, including 60 in the radiotherapy group and 52 in the chemotherapy group. Table 4 displays the sites of first recurrence for each treatment cohort. Patients with multiple sites of first recurrence are tabulated once for each recurrence site listed in this table. Among 206 study patients, the sites of first recurrence for the WAI group included the vagina in 3.8% patients, pelvis in 13.3% patients, abdomen in 27.6% patients, and distant sites in 25.7% patients. The corresponding sites of first recurrence for the CIM group included the vagina in 9.9% patients, pelvis in 13.8% patients, abdomen in 18.8% patients, and other distant sites in 23.8% patients. There were slightly more vaginal recurrences in the CIM group and more abdominal recurrences in the WAI group, but these differences were not statistically significant.
Table 4.
Patterns of Failure
| WAI Regimen (n = 105) |
CIM Regimen (n = 101) |
|
|---|---|---|
| Sites of Recurrence* | Number of Cases | Number of Cases |
| Vagina | 4 | 10 |
| Pelvis | 14 | 14 |
| Abdomen | 29 | 19 |
| Lung | 14 | 14 |
| Other Distant Sites | 13 | 10 |
Abbreviations: WAI, whole abdominal irradiation; CIM, cisplatin, ifosfamide with mesna chemotherapy.
n = total number of cases in each arm
Some patients had multiple sites of relapse.
Time to Recurrence and Overall Survival
At the time of this analysis the median duration of follow-up for patients alive at last contact was five years and three months. There were 38 patients in the radiotherapy arm alive at last contact compared to 46 patients in the chemotherapy arm. There were 12 patients in the WAI group and eight in the CIM group who died without any intervening evidence of recurrence. However, most of the other deaths were attributed to progressing cancer.
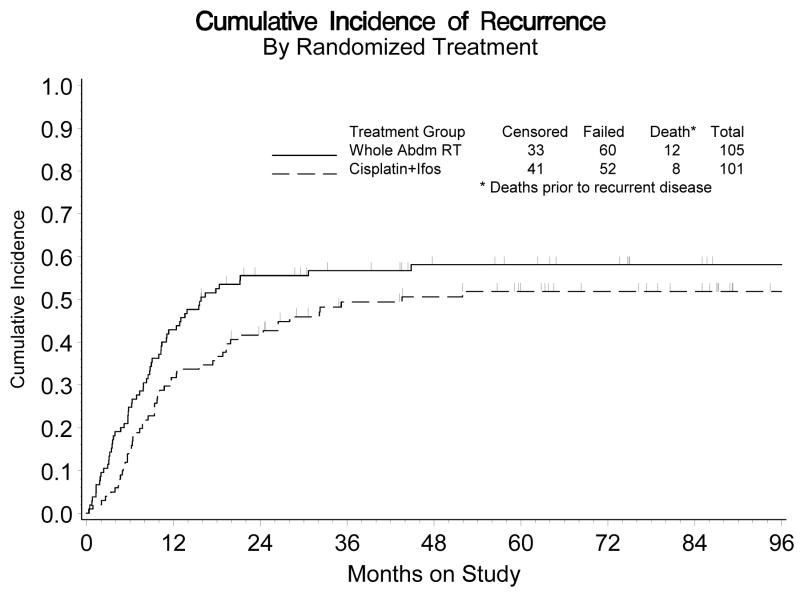
Figure 2 shows the estimated cumulative incidence of recurrence for each treatment group. For both groups, the recurrence rate was relatively high during the first three years following diagnosis, but thereafter it markedly subsided. The estimated crude probability of relapse within five years was 58% vs 52% for the WAI and CIM groups, respectively. After adjusting for stage of disease and age at diagnosis, the estimated recurrence rate for those randomized to CIM was 29% lower than those patients who were randomized to WAI (Relative Hazard [RH]= 0.789, 95% confidence interval [CI]: 0.530 – 1.176). This difference was not statistically significant (p = 0.245, 2-tail test).
Figure 2.
Cumulative Incidence of Recurrence by Randomized Treatment
Abbreviations: Abdm, abdominal; ifos, ifosfamide.
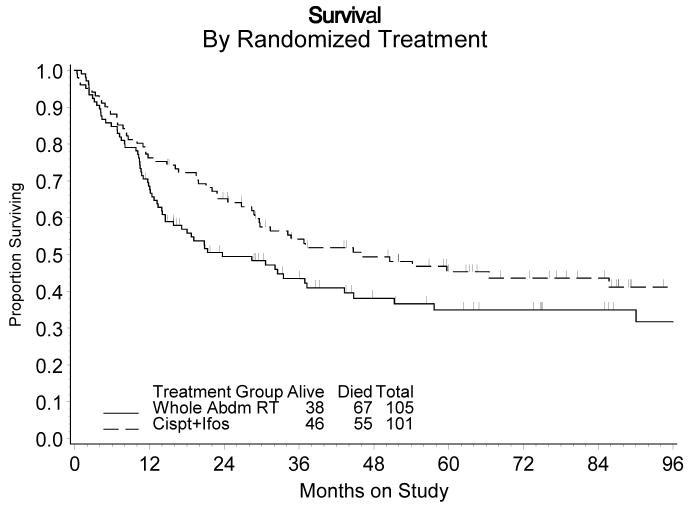
Figure 3 displays the estimated cumulative distribution of the survival time for each treatment group. The estimated crude probability of surviving at least five years following diagnosis was approximately 35% for those randomized to WAI versus 45% for those randomized to CIM. After adjusting for stage and age at diagnosis, the estimated death rate was 29% lower for CIM patients than WAI patients (RH = 0.712, 95% CI: 0.484 – 1.048, p = 0.085, 2-tail test). A similar result was obtained when all patients (regardless of eligibility) were included in the analysis of survival for an intention-to-treat analysis (RH=0.727, 95% CI: 0.503 – 1.050, p=0.089).
Figure 3.
Survival by Randomized Treatment
Abbreviations: Abdm, abdominal; ifos, ifosfamide.
An exploratory analysis suggests that the treatment relative recurrence rates may vary over time. That is, an assessment of heterogeneity of the treatment effect comparing the relative recurrence rates within the first 18 months of diagnosis vs thereafter, adjusted for stage and age (χ21 df = 4.25, p = 0.039), suggests that the recurrence rate was lower for those in the CIM group during the first 18 months (RH=0.631, 95% CI: 0.402 – 0.990), but then this advantage wanes. This observation may indicate that CIM delays the onset of recurrence for those destined to recur; however, chemotherapy does not appreciably alter the overall proportion that will ultimately recur.
Finally, the median time to death following disease recurrence was 4 months and only 25% of those who recurred survived longer than one year following recurrence.
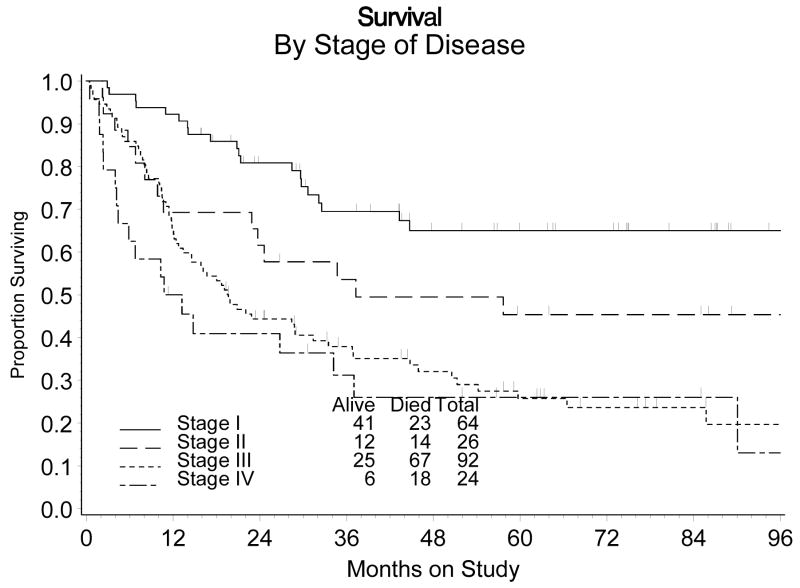
Prognostic Factors
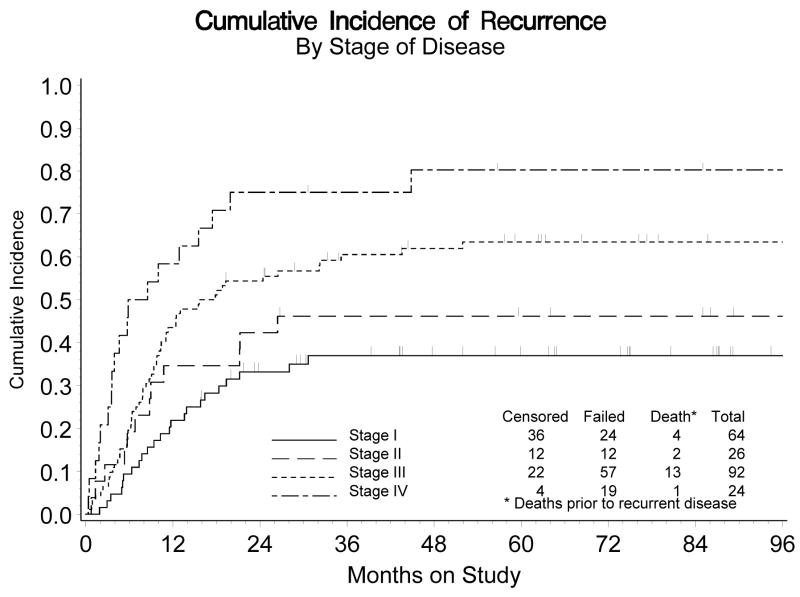
The estimated probability of recurring within five years for those diagnosed with stage I, II, III, or IV disease is: 37%, 46%, 63% and 80%, respectively (Figure 4). The differences in recurrence rates among these groups of patients was statistically significant (p<0.001). Similarly, there was a greater death rate among those patients diagnosed with more advanced disease (stage III or IV). The estimated probability of surviving five years or more was 65%, 45%, 26% and 26% for those with stage I, II, III or IV disease, respectively. Overall, the death rate was 2.7 times greater for those diagnosed with the more advanced stage (III or IV) disease (RH: 2.70, 95% CI: 1.83 – 3.99). An assessment for heterogeneity of the treatment effect across the patients distinguished by the stage of their disease (I and II vs III and IV) was not statistically significant for either recurrence (χ21 df = 0.083, p = 0.773) or survival (χ21 df = 1.37, p = 0.242).
Figure 4.
Cumulative Incidence of Recurrence by Stage of Disease
Of the 11 patients with gross residual disease, all but two patients (one in each treatment group) experienced recurrent disease. The first refused further follow-up five years after enrolling onto the study and the second is alive and well after seven years of follow-up. The median time to recurrence for those with gross residual disease was 10 months.
Whereas an estimated 48% of the patients who are younger than 65 at the time of diagnosis experienced a recurrence within five years, 62% of those patients older than 65 recurred within five years. The relapse rate was 56% greater among the older subgroup of patients (RH: 1.56, 95% CI: (1.07 – 2.28, p=0.020). Likewise, the younger patients experienced longer survival. Whereas, 50% of those younger than 65 live five years or more, only 30.6% of the older patients do so. The adjusted death rate was also higher for the older patients (RH: 1.77, 95% CI: 1.23 – 2.55, p<0.003). An assessment for heterogeneity of the treatment effect across these age groups was not statistically significant for either recurrence (χ21 df = 1.24, p = 0.265) or survival (χ21 df = 0.52, p = 0.470).
The adjusted recurrence rate for patients with initial performance status 0 was very similar to the recurrence rate for those with either performance status 1 or 2 (RH: 1.02, 95% CI: 0.687–1.50, p=0.93)
The risk of recurrence and death was higher among patients who identified themselves as Black compared to White. Relative to Whites, the recurrence rate, adjusted for stage, age and treatment, was 59% higher (RH: 1.59, 95% CI: 1.03 – 2.44, p=0.034) and the overall death rate was 26% higher (RH: 1.26, 95% CI: 0.832 – 1.92, p=0.27). An assessment for heterogeneity of the treatment effects across Black and White racial groups was not statistically significant for recurrence (χ21 df = 0.32, p = 0.568) or survival (χ21 df = 0.41, p = 0.522)
Adjusted for stage of disease, age group and randomized treatment, the recurrence rate for patients diagnosed with heterologous tumors was only 6% greater than those diagnosed with homologous tumors (RH=1.06, 95% confidence interval: (0.720 – 1.55), p=0.78).
DISCUSSION
There has been no available data from prospectively randomized Phase III trials concerning the role of adjuvant radiation therapy with or without chemotherapy in the management of patients with CS of the uterus until the Gynecological Cancer Group (GCG) of the European Organization for Research and Treatment of Cancer (EORTC) published the results of Protocol 55874. This study focused on patients with surgical Stage I and II uterine sarcomas including 103 with leiomyosarcoma, 91 with carcinosarcoma, and 28 with endometrial stromal sarcoma. Similar to GOG 150 (the subject of this paper), EORTC-GCG 55874, which opened in 1987, required an extensive time interval (13 years) to reach its targeted accrual of 224 patients. Study patients were randomized to either pelvic EBRT or observation. An updated analysis has shown 14% experienced local relapses in the adjuvantly treated cohort versus 24% in the observed group (p = 0.004).16 However, adjuvant treatment had no significant impact on either progression-free or OS.
However, there are some significant differences between GOG 150 and EORTC-GGC 55874. The former focused only on uterine CS, while the latter additionally included leiomyosarcomas and endometrial stromal sarcoma. Furthermore, GOG 150 evaluated all stages of disease (as long as there was no extra-abdominal tumor spread) rather than just stages I and II, as was evaluated in the European study. Also, GOG 150 subdivided sites of disease relapse as pelvic, abdominal, and distant. Yet, the EORTC study combined abdominal and distant recurrences as one site (distant), perhaps obscuring important information concerning the effectiveness of adjuvant pelvic EBRT in patients with uterine sarcomas.
There have been several retrospective reviews of patients with US that have included uterine CS evaluating the effect of adjuvant therapy.17,18,19,20,21,22,23,24,25,26,27 Those that have involved postoperative pelvic EBRT have shown a consistent decrease in pelvic failures but no significant impact on overall patient survival.17, 19, 20, 21, 22, 23, 24, 27 However, two retrospective studies did claim an OS benefit with the addition of adjuvant pelvic irradiation for patients with surgical stage I and II disease.25, 26
When comparing the patterns of failure between WAI and CIM in GOG-150 for stages I/II/III/IV, only vaginal recurrences appeared to be increased in the chemotherapy cohort versus the radiotherapy cohort. In fact, all of the other sites of relapse were similar except for a notable reduction in abdominal failures in the CIM treated group as compared with the WAI treated arm. Yet, there was a significant increase in serious late adverse events in those who underwent postoperative radiotherapy. Based on these patterns of recurrence future studies may consider combining vaginal brachytherapy which has markedly less risk of late effects (especially in the gastrointestinal tract), and at least three cycles of chemotherapy.
The most effective chemotherapeutic regimen for advanced stage uterine carcinosarcomas remains unclear at this point. Recent studies have shown that ifosfamide-based combination chemotherapy significantly improved PFS and OS over ifosfamide alone in advanced, persistent, or recurrent uterine carcinosarcoma.28,29 Sutton et al. showed a benefit regarding PFS when adding cisplatin to ifosfamide/mesna regimen in GOG 108.28 In addition, Homesley et al. have recently demonstrated the OS benefit in this group of patients when paclitaxel was added to ifosfamide/mesna in GOG 161.29 However, all patients treated in both protocols received 8 cycles of either single agent ifosfamide or combination chemotherapy for their advanced, persistent or recurrent uterine carcinosarcoma.
Our current study treated all stages, including stage III and IV, with 3 cycles of CIM that might raise the question of possible “undertreatment” of this particularly high-risk group of patients. However, it must be pointed out that when the concept that led to GOG 150 was initially proposed and debated approximately 15 years ago, and then opened to accrual in December 1993, most investigators at the time only felt comfortable with 3 cycles of CIM. In addition, the selected chemotherapy regimen for this study was based on the already completed GOG trial involving cisplatin and ifosfamide as stated in the Introduction section of this paper. Certainly with more experience and advance in chemotherapeutic management, we can begin not only to plan the administration of more cycles of chemotherapy but also to consider other new combination regimens.
This study on adjuvant therapy for stages I, II, III, and IV uterine CS with less than 1 cm residual disease indicates that stage of disease and age at diagnosis are significant predictors of patient outcome. Moreover, GOG 150 has demonstrated that the recurrence rates are significantly higher among Black patients when compared to White. Since it has also been shown that there is an increased incidence of uterine CSs in Black versus White patients, this latter finding merits further investigation into a possible connection.30,31
This study was not able to demonstrate a significant difference in relapse rate or OS between CIM and WAI, perhaps in part because of the relatively small sample size. This is, however, the largest randomized adjuvant trial we are aware of in uterine CSs. Completing a larger Phase III study within a reasonable time frame would be very problematic in this rare tumor type, even with extensive national inter-group collaboration. Therefore, one is obliged to extract from this trial indications of how to proceed, recognizing also that neither approach has been compared to observation. CIM appeared to have a slight advantage and was not more toxic. Potentially more effective regimens are now available, using longer treatment and/or containing paclitaxel. Also, an exploratory analysis suggested an early but unsustained effect of chemotherapy that conceivably could be enhanced by longer treatment. Due to the high relapse rate and poor OS, the imperative for more effective postoperative strategies for this patient population remains.
Figure 5.
Survival by Stage of Disease
Acknowledgments
The authors wish to thank Drs. Nick Spirtos and William H. Rodgers for reviewing this manuscript. Both of these individuals were instrumental in the review process, especially in helping to clarify background clinical and pathological information for study patients.
This study was supported by the National Cancer Institute Grants No. CA27469 to the GOG Administrative Office and CA37517 to the GOG Statistical and Data Center.
The following Gynecologic Oncology Group member institutions participated in this study: University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, SUNY Downstate Medical Center, University of Kentucky, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Women’s Cancer Center, University of Oklahoma, University of Virginia Health Sciences Center, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Case Western Reserve University, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Ellis Fischel Cancer Center, University of Arkansas Medical Center and Community Clinical Oncology Program.
Footnotes
Presented in part at the 42nd Annual Meeting of the American Society of Clinical Oncology, Atlanta, GA, June 2–6, 2006 and at the 11th Biennial Meeting of the International Gynecologic Cancer Society, Santa Monica, CA, October 14–18, 2006.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1. [November 10, 2006]; http://www.cancer.org/docroot/home/index.asp.
- 2.Curtin JP, Kavanagh JJ, Fox H, et al. Corpus: Mesenchymal tumors. In: Hoskins WJ, Perez CA, Young RC, editors. Principles and Practice of Gynecologic Oncology. 3. Lippincott, Williams & Wilkins; Philadelphia: 2000. p. 961. [Google Scholar]
- 3.Menczer J, Levy T, Piura B, et al. A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecol Oncol. 2005;97:166–70. doi: 10.1016/j.ygyno.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 4.McCluggage WG. Uterine carcinosarcomas (malignant mixed Mullerian tumors) are metaplastic carcinosarcomas. Int J Gynecol Cancer. 2002;12:687–90. doi: 10.1136/ijgc-00009577-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 5.WHO. Pathology and genetics of tumors of the breast and female genital organs. Lyon: IARC Press; 2003. [Google Scholar]
- 6.Sutton G, Kavanagh J, Wolfson AH, et al. Corpus: Mesenchymal tumors. In: Hoskins WJ, Perez CA, Young RC, Barakat RR, Markman M, Randall ME, editors. Principles and Practice of Gynecologic Oncology. 4. Lippincott, Williams & Wilkins; Philadelphia: 2004. p. 883. [Google Scholar]
- 7.Wolfson AH, Wolfson DH, Sittler SY, et al. A multivariate analysis of clinicopathologic factors for predicting outcome in uterine sarcomas. Gynecol Oncol. 1994;52:56–62. doi: 10.1006/gyno.1994.1011. [DOI] [PubMed] [Google Scholar]
- 8.Omura GA, Blessing JA, Major FJ, et al. A randomized clinical trial of adjuvant adriamycin in uterine sarcomas: A Gynecologic Oncology Group study. J Clin Oncol. 1985;3:1240–45. doi: 10.1200/JCO.1985.3.9.1240. [DOI] [PubMed] [Google Scholar]
- 9.Hornback NB, Omura GA, Major FJ. Observations on the use of adjuvant radiation therapy in patients with stage I and II uterine sarcoma. Int J Radiat Oncol Biol Phys. 1986;12:2127–30. doi: 10.1016/0360-3016(86)90011-8. [DOI] [PubMed] [Google Scholar]
- 10.Major FJ, Blessing JA, Silverberg SG, et al. Prognostic factors in early stage uterine sarcomas: A Gynecologic Oncology study. Cancer. 1993;71:1702–9. doi: 10.1002/cncr.2820710440. [DOI] [PubMed] [Google Scholar]
- 11.Sutton G, Kauderer J, Carson LF, et al. Adjuvant ifosfamide and cisplatin in patients with completely resected stage I or II carcinosarcomas (mixed mesodermal tumors) of the uterus: a Gynecologic Oncology Group Study. Gynecol Oncol. 2005;96:630–4. doi: 10.1016/j.ygyno.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–81. [Google Scholar]
- 13.Korn EL, Dorey FJ. Applications of Crude Incidence Curves. Stats in Med. 1992;11:813–29. doi: 10.1002/sim.4780110611. [DOI] [PubMed] [Google Scholar]
- 14.Cox DR. Regression model and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 15.Blessing JA. Design, analysis and interpretation of chemotherapy trials in gynecology cancer. In: Deppe G, editor. Chemotherapy of Gynecology Cancer. 2. New York, NY: Alan R Liss Inc; 1990. pp. 63–97. [Google Scholar]
- 16.Reed NS, Mangioni C, Malmstrom H, et al. Phase III randomized study to evaluate the role of adjuvant pelvic radiotherapy in the treatment of uterine sarcomas stages I and II. An EORTC Gynaecological Cancer Group study (protocol 55874) doi: 10.1016/j.ejca.2008.01.019. (Manuscript in preparation) [DOI] [PubMed] [Google Scholar]
- 17.Dusenbery KE, Potish RA, Argenta PA, et al. On the apparent failure of adjuvant pelvic radiotherapy to improve survival for women with uterine sarcomas confined to the uterus. Am J Clin Oncol. 2005;28:295–300. doi: 10.1097/01.coc.0000156919.04133.98. [DOI] [PubMed] [Google Scholar]
- 18.Menczer J, Levy T, Piura B, et al. A comparison between different postoperative treatment modalities of uterine carcinosarcoma. Gynecol Oncol. 2005;97(1):166–70. doi: 10.1016/j.ygyno.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Dusenbery KE, Potish RA, Judson P. Limitations of adjuvant radiotherapy for uterine sarcomas spread beyond the uterus. Gynecol Oncol. 2004;94:191–96. doi: 10.1016/j.ygyno.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Callister M, Ramondetta LM, Jhingran A, et al. Malignant mixed Mullerian tumors of the uterus: Analysis of patterns of failure, prognostic factors, and treatment outcome. Int J Radiat Oncol Biol Phys. 2004;58:786–96. doi: 10.1016/S0360-3016(03)01561-X. [DOI] [PubMed] [Google Scholar]
- 21.Gadducci A, Romanini A. Adjuvant chemotherapy in early stage uterine sarcomas: An open question. Gynaecol Oncol. 2001;22:352–7. [PubMed] [Google Scholar]
- 22.Le T. Adjuvant pelvic radiotherapy for uterine carcinosarcoma in a high risk population. Eur J Surg Oncol. 2001;27:282–5. doi: 10.1053/ejso.2000.1104. [DOI] [PubMed] [Google Scholar]
- 23.Knocke TH, Weitmann HD, Kucera H, et al. Results of primary and adjuvant radiotherapy in the treatment of mixed Mullerian tumors of the corpus uteri. Gynecol Oncol. 1999;73:389–95. doi: 10.1006/gyno.1999.5400. [DOI] [PubMed] [Google Scholar]
- 24.Chauveinc L, Deniaud E, Plancher C, et al. Uterine sarcomas: The Curie Institut experience: Prognosis, factors and adjuvant treatment. Gynecol Oncol. 1999;72:232–7. doi: 10.1006/gyno.1998.5251. [DOI] [PubMed] [Google Scholar]
- 25.Molpus KL, Redline-Frazier S, Reed G, et al. Postoperative pelvic irradiation in early stage uterine mixed Mullerian tumors. Gynaecological Oncol. 1998;19:541–6. [PubMed] [Google Scholar]
- 26.Gerszten K, Faul C, Kounelis S, et al. The impact of adjuvant carcinosarcoma of the uterus. Gynecol Oncol. 1998;68:8–13. doi: 10.1006/gyno.1997.4901. [DOI] [PubMed] [Google Scholar]
- 27.Chi DS, Mychalczak B, Saigo PE, et al. The role of whole-pelvic irradiation in the treatment of early-stage uterine carcinosarcoma. Gynecol Oncol. 1997;65:493–8. doi: 10.1006/gyno.1997.4676. [DOI] [PubMed] [Google Scholar]
- 28.Sutton G, Brunetto VL, Kilgore L, et al. A phase III trial of ifosfamide with or without cisplatin in carcinosarcinoma of the uterus: A Gynecologic Oncology Group study. Gynecol Oncol. 2000;79:147–53. doi: 10.1006/gyno.2000.6001. [DOI] [PubMed] [Google Scholar]
- 29.Homesley HD, Filiaci V, Markman M, et al. Phase III trial of ifosfamide with or without paclitaxel in advanced uterine carcinosarcoma: A Gynecologic Oncology Group study. J Clin Oncol. 2007;25:526–31. doi: 10.1200/JCO.2006.06.4907. [DOI] [PubMed] [Google Scholar]
- 30.Amant F, Dreyer L, Makin J, et al. Uterine sarcomas in South African black women: A clinicopathologic study with ethnic considerations. Eur J Gynaecol Oncol. 2001;22:194–200. [PubMed] [Google Scholar]
- 31.Brooks SE, Zhan M, Cote T, et al. Surveillance, epidemiology, and end results analysis of 2677 cases of uterine sarcoma. Gynecol Oncol. 2004;93:204–208. doi: 10.1016/j.ygyno.2003.12.029. [DOI] [PubMed] [Google Scholar]