Abstract
Background
Recent data have implicated telomere length shortening as a potential risk predictor for cardiovascular disease. However, to date, prospective epidemiological data are scarce.
Methods and Results
Using leukocyte DNA samples collected at baseline in a prospective cohort of 14,916 initially healthy American men, we examined the relationship of mean telomere repeat copy number to single gene copy number (T/S ratio), using a re-modified quantitative polymerase chain reaction protocol, amongst 337 white males who subsequently developed an incident myocardial infarction (MI), and amongst an equal number of age- and smoking-matched white males who remained free of reported vascular disease during follow-up (controls). The mean follow-up time since randomization was 3.85 years. The T/S ratio was inversely correlated with age in the controls (R=-0.114; p=0.036). The loge-transformed T/S ratios were significantly smaller in the MI cases (3.41±0.63) than the MI controls (3.52±0.78) (p=0.01). In a multi-variable adjusted analysis, decreased T/S ratio was significantly associated with risk of MI (odds ratio=1.621; 95%CI=1.140-2.304; p=0.007).
Conclusions
In summary, the present investigation has shown an association of telomere length shortening with increased risk of incident myocardial infarction, further suggesting the importance of telomere biology in atherogenesis.
Keywords: relative mean telomere length, MI, risk factor
Introduction
Vascular disorders, including myocardial infarction (MI), are leading causes of mortality and morbidity in modern societies (1, 2). The underlying patho-physiology is likely to be under the influence of both genetic and environmental factors.
Telomeres are tandem repeats of DNA sequences —special chromatin structures— located at the ends of eukaryotic chromosomes. One function of these structures is to protect the telomeric regions from recombination and degradation, thus avoiding a DNA damage cellular response (3). Recent evidence has demonstrated the relevance of telomere biology in human disorders including cardiovascular disease (3). In cross-sectional, case-control, and prospective studies, shortening of telomere length has recently been associated with chronic heart failure (4), degenerative aortic valve stenosis (5), coronary artery diseases (6-8), and premature MI (9).
The standard method, Southern blot analysis, for determining telomere length in human genomic DNA is both labor-intensive and time-consuming. Recently, a simple, rapid and validated method, using quantitative polymerase chain reaction (qPCR), has been developed and commonly used for relative telomere length measurement (10). As described previously, this qPCR approach requires amplifying the same DNA sample in two separate yet different PCR conditions on two separate reaction plates: one targeting a single-copy gene, the other the telomeric repeat region. As such, two separate standard curves with serial dilution of a reference DNA sample is needed for normalization (10).
We, therefore, (i) re-modified the Cawthon's method (10) and developed a unified qPCR protocol, in which all reactions were done under the same condition in a single plate, hence reducing inter-plate assay variation, and eliminating the use of a standard curve for normalization, and (ii) prospectively examined the possible association of mean telomere length with incident MI using a nested, matched case-control sample drawn from the prospective Physicians' Health Study (PHS) cohort.
Materials and Methods
Study Design
We employed a nested case-control design within the PHS, a randomized, double-blinded, placebo-controlled trial of aspirin and beta-carotene initiated in 1982 among 22,071 male, predominantly white (>94%), U.S. physicians, 40 to 84 years of age at study entry (11). Before randomization, 14,916 participants provided an EDTA-anticoagulated blood sample that was stored for genetic analysis. All participants were free of prior myocardial infarction (MI), stroke, transient ischemic attacks, deep venous thrombosis/pulmonary embolism (DVT/PE) and cancer at study entry. Yearly follow-up self-report questionnaires provide reliable updated information on newly developed diseases and the presence or absence of other cardiovascular risk factors. History of cardiovascular risk factors, such as smoking status (never, past, current), history of hypertension (≥140/90 mmHg or on anti-hypertensive therapy), presence or absence of diabetes or hyperlipidemia (≥240 mg/dL), was defined by self-report of diagnosis at entry into the study (baseline). For all reported incident vascular events occurring after study enrollment, hospital records, death certificates, and autopsy reports were requested and reviewed by an end points committee using standardized diagnostic criteria.
The diagnosis of MI was confirmed by evidence of symptoms in the presence of either diagnostic elevations of cardiac enzymes or diagnostic changes on electrocardiograms. In the case of fatal events, the diagnosis of MI was also accepted based on autopsy findings (11). The mean length of follow-up since randomization was 3.85 years.
For each case, a control matched by age±2 years, smoking history (never, past, or current) and length of follow-up were chosen among those subjects who remained free of vascular diseases; 341 case-control pairs were identified. The present association study consisted of white men only. The study was approved by the Brigham and Women's Hospital Institutional Review Board for Human Subjects Research.
Mean Telomere Length Determination
‘Unified’ Quantitative Polymerase Chain Reaction Assay
Genomic DNA was extracted from whole blood using the QIAmp Spin Column protocol (Qiagen, Chatsworth, CA). Telomere length was determined by a ‘unified’ version modified from the quantitative polymerase chain reaction (qPCR) protocol previously described by Cawthon RM (10). The differences of the present protocol from the Cawthon's protocol are that (i) both telomere and single-copy gene amplifications on each DNA sample are performed, side by side, on the same reaction plate, and (ii) amplifications are carried out under the same PCR conditions, thus eliminating the inter-plate variation (and the use of standard curve). Because the Ct values for each PCR reaction for each sample are measured on the same plate, any small variation in the threshold at which the Ct is measured is likely to have only a very small effect on the T/S ratio. This is in contrast to the original Cawthon's method, in which variation in the threshold between plates could have a substantial effect on the delta Ct.
In brief, two master mixes of PCR reagents were prepared, one for telomere reaction and one for single-copy gene reaction (36B4 on chromosome 12). Telomere repeat copy number to single gene copy number (T/S) ratio was determined on an ABI7900HT Sequence Detection System (Applied Biosystems, Foster City, CA) in a 384-well format using the following PCR protocol: 95°C for 15 minutes to activate Taq polymerase; 40 cycles of denaturation at 95°C for 15 seconds, and annealing-extension at 54°C for 2 minutes. Each 15uL amplification reaction volume contained 1× Qiagen Quantitect Sybr Green Master Mix (Qiagen, Chatsworth, CA) and 10ng of template DNA. The primer sequences used were those described previously (10). The final primer concentrations were: tel 1, 300nM; tel 2, 900nM; 36B4u and 36B4d, each 400nM. All samples for both the telomere and single-copy gene amplifications were done in duplicate on the same 384-well plate. Duplicates of a no-template control were included in each run. Ct-value assignment was carried out by two independent observers, and if necessary, a complete re-genotyping was performed. Melting (dissociation) curve analysis was performed on every run to verify specificity and identity of the PCR products. Ct values were determined in the exponential phase. The Ct values generated were used to calculate the T/S ratio for each sample using the equation: T/S=2-ΔCt (where ΔCt=Ct single-copy gene-Ct telomere). In four subjects, we encountered difficulties in amplifications; these samples along with the matched counterparts were excluded from the analysis. T/S ratios were determined on 337 case-control pairs. Results were scored blinded as to case-control status.
Protocol Comparison and Quality Control Assessment
The comparability between the present qPCR protocol and the Cawthon's method (10) was tested on 96 samples randomly selected and analyzed in duplicate. For internal reproducibility of our unified protocol, we again randomly selected 96 samples and repeated the present PCR protocol in different well-positioning on a different day. The comparability to the Cawthon's method and the internal reproducibility of our present qPCR method were: Spearman correlation=0.670, p<0.0001; and Spearman correlation=0.711, p<0.0001, respectively.
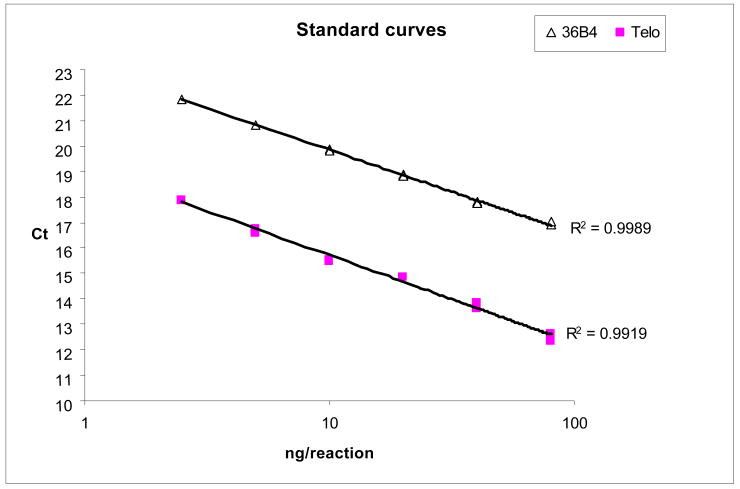
The coefficient of variation of the telomere, single-gene, and T/S ratio duplicate assays were <4%, <2%, and <5%, respectively. Furthermore, for comparison purposes, we performed standard curves with serial dilution (similar to that described by Cawthon) using our present amplification protocol; the correlation coefficients for the telomere and single-gene standard curves were R2=-0.99 and R2=-1.00, respectively; demonstrating the robustness of our present modified protocol (see Figure). In addition, these data demonstrate the amplification linearity of the qPCR performance for the telomere assays and the single gene assays over a range of input DNA amount, and that this range is substantially larger than the range of input DNA used for the actual experiments reported. Since the use of a calibrator (normalization to a reference DNA sample) was described in the Cawthon's PCR protocol, we thus performed using the same 96 randomly selected samples two PCR runs on different days (one without incorporating a calibrator, and the other with and normalized to a calibrator), and a significant correlation of T/S ratios between runs was again observed (data not shown), further demonstrating the robustness of the current ‘unified’ protocol.
Statistical Analysis
As previously noted, the observed telomere-repeat copy number to single gene copy number ratios (T/S ratios) had a skewed distribution, the data were loge-transformed. The T/S ratios between cases and controls were compared using the paired t-test. Spearman's correlation analysis was used to assess the effects of age, body-mass index (BMI), blood pressure, and C-reactive protein plasma concentrations on relative T/S ratios amongst the controls. Relative risk of MI associated with log-transformed T/S ratios were calculated separately by conditional logistic regression analysis, matched on age, smoking status, and length of follow-up since randomization, and further controlling for randomized treatment assignment, history of hypertension (≥140/90 mmHg or on anti-hypertensive medications), presence or absence of diabetes, BMI and hyperlipidemia (≥6.21 mmol/L or ≥240 mg/dL). All analyses were carried out using SAS/Genetics 9.1 package [SAS Institute Inc., Cary, NC]. A two-tailed p-value of 0.05 was considered a statistically significant result.
Results
Baseline characteristics of the study participants are shown in Table 1. As expected, the case subjects had a higher prevalence of traditional vascular risk factors at baseline than did the control subjects. The median (interquartile range) of the observed T/S ratios for the controls and the cases were 30.0 (22.8-45.1), and 29.1 (21.5-41.0), respectively. The observed T/S ratios were significantly smaller in the MI cases than the MI controls (p=0.01; Table 1). An inverse relationship between the observed T/S ratios and age in our controls was also found (Spearman correlation=−0.114, p=0.036); however, no correlation was observed for current smoking, BMI, blood pressure, or CRP plasma levels (all p>0.20; Supplementary Data Table 1). Furthermore, a significant association of decreased T/S ratios with an increased risk of incident MI was found in a crude or an adjusted regression analysis (Table 2).
Table 1.
Baseline characteristics of study participants.
| MI-Controls (N=337) | MI-Cases (N=337) | p | |
|---|---|---|---|
| Age (years) | 60.1±8.7 | 60.2±.8.7 | m.v. |
| Smoking Status (%) | m.v. | ||
| Never | 43.1 | 43.1 | |
| Past | 41.4 | 41.4 | |
| Current | 15.5 | 15.5 | |
| Body-mass Index, kg/m2 | 24.9±2.9 | 25.5±3.1 | 0.02 |
| Hyperlipidemia ≥240 mg/dL (%) | 14.5 | 25.2 | 0.001 |
| Systolic Blood Pressure, mmHg | 127.9±12.3 | 131.1±13.7 | 0.001 |
| Diastolic Blood Pressure, mmHg | 79.4±7.1 | 81.2±8.2 | 0.002 |
| Hypertension (%) | 27.1 | 44.1 | <0.0001 |
| * C-reactive Protein, mg/L | 1.2 [ 0.6-2.1] | 1.6 [0.9-2.6] | 0.005 |
| Diabetes (%) | 3.2 | 6.8 | 0.04 |
| Aspirin use (%) | 44.3 | 41.4 | 0.46 |
| Family History of Premature CAD <60 years (%) | 9.5 | 12.4 | 0.21 |
| Loge-transformed T/S ratio | 3.52±0.78 | 3.41±0.63 | 0.01 |
Mean±SD unless otherwise stated.
MI=myocardial infarction, CAD=coronary artery disease, T/S=Telomere repeat copy number to single gene copy number, m.v.=matched variable.
Continuous and categorical variables were tested by paired t-test and McNemar's test, respectively.
Median and interquartile range.
Table 2.
Conditional logistic regression analysis of shortening loge-transformed mean telomere length.
| Crude | Adjusted | |
|---|---|---|
| OR, 95%CI, p | OR, 95%CI, p | |
| MI | 1.497; 1.098-2.041; 0.011 | 1.621; 1.140-2.304; 0.007 |
Crude=conditional on age, smoking status, time of follow-up.
Adjusted=further controlling for randomized treatment group, body-mass index, hypertension, diabetes, and hyperlipidemia.
MI=myocardial infarction.
Discussion
The present prospective, nested, matched case-control investigation examined the relationship of mean telomere length with risk of incident MI, and has shown that participants with decreased T/S ratio at baseline were significantly associated with increased risk of incident MI. Our findings are in concordance with previous observations in subjects with premature MI (9), and with coronary heart disease (7). The present study also found an inverse correlation between T/S ratios and age-at-baseline in the control participants. Hence, our findings further support the notion that telomere biology (and the associated biological aging) contributes to the risk of cardiovascular disease.
We presented a modified qPCR protocol for mean telomere length measurement using a single amplification condition, and have demonstrated its comparability and reproducibility. As stated previously, our observation of an inverse relationship of T/S ratios and age is in concordance with previous studies (7, 9, 12), thus demonstrating the reliability of the present qPCR method in determining mean telomere length.
Taken altogether, the present investigation and previous studies (4-9) have implicated the importance of leukocyte telomere biology in cardiovascular disease) further suggest that telomere biology plays an important role in the underlying pathophysiology of atherosclerosis. As noted previously, whether telomere shortening at baseline is merely another surrogate or whether the association does carry a functional mechanism to atherogenesis remains to be determined (7).
The nature of the present investigation in which the determination of a case status was based solely on the subsequent development of disease rather than on any arbitrary selection criteria designed by the investigators, greatly reduce the possibility of bias and confounding. Nonetheless, our study population consists of white males so the data cannot be generalized to other ethnic groups, women, and populations. Furthermore, the possibility that an unmeasured factor could account for the observed association of telomere length with the risk of MI cannot be excluded, especially when few cardiovascular risk factors' association with telomere length was examined (for example, triglycerides, LDL cholesterol, HDL cholesterol, glucose and homocysteine were not included). As telomere biology represents a new and challenging research field, further thoughts on future development of qPCR technique (for example, different multiplex possibilities, the use of synthetic reference DNA, etc.) with comparison to the gold-standard Southern Blot method is worthwhile.
In conclusion, in this prospective, nested case-control study of middle-aged white US men, we found an association of leukocyte telomere length shortening with increased risk of incident MI.
Supplementary Material
Figure. Standard curves: six DNA concentrations were prepared by serial dilution (dilution factor 2) with the final amounts per reaction ranged from 2.5 to 80 ng. The Ct values in duplicate for Telo were: [17.84/17.88], [16.60/16.76], [15.52/15.48], [14.83/14.85], [13.63/13.86], and [12.35/12.64]. The Ct values in duplicate for 36B4 were: [21.84/21.80], [20.83/20.80], [19.87/19.83], [18.86/18.81], [17.78/17.77], and [16.89/17.00].
Acknowledgments
Funding sources: Supported by grants from the National Heart Lung and Blood Institute (HL-58755, HL-63293), the Leducq Foundation, Paris, France, the American Heart Association, and the Donald W. Reynolds Foundation, Las Vegas, Nevada.
Footnotes
Authorship: Contribution: R.Y.L.Z., S.E.M. and S.G. designed research, performed research, analyzed data. All authors contributed to data interpretation and manuscript preparation. All authors approved the manuscript as its present form.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
References
- 1.Forouhi NG, Sattar N. CVD risk factors and ethnicity--a homogeneous relationship? Atheroscler Suppl. 2006;7:11–9. doi: 10.1016/j.atherosclerosissup.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Primatesta P, Allender S, Ciccarelli P, Doring A, Graff-Iversen S, Holub J, et al. Cardiovascular surveys: manual of operations. Eur J Cardiovasc Prev Rehabil. 2007;14 3:S43–61. doi: 10.1097/01.hjr.0000277988.18096.3b. [DOI] [PubMed] [Google Scholar]
- 3.Fuster JJ, Andres V. Telomere biology and cardiovascular disease. Circ Res. 2006;99:1167–80. doi: 10.1161/01.RES.0000251281.00845.18. [DOI] [PubMed] [Google Scholar]
- 4.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, et al. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–64. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 5.Kurz DJ, Kloeckener-Gruissem B, Akhmedov A, Eberli FR, Buhler I, Berger W, et al. Degenerative aortic valve stenosis, but not coronary disease, is associated with shorter telomere length in the elderly. Arterioscler Thromb Vasc Biol. 2006;26:e114–7. doi: 10.1161/01.ATV.0000222961.24912.69. [DOI] [PubMed] [Google Scholar]
- 6.Ogami M, Ikura Y, Ohsawa M, Matsuo T, Kayo S, Yoshimi N, et al. Telomere shortening in human coronary artery diseases. Arterioscler Thromb Vasc Biol. 2004;24:546–50. doi: 10.1161/01.ATV.0000117200.46938.e7. [DOI] [PubMed] [Google Scholar]
- 7.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, et al. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–14. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 8.Fitzpatrick AL, Kronmal RA, Gardner JP, Psaty BM, Jenny NS, Tracy RP, et al. Leukocyte telomere length and cardiovascular disease in the cardiovascular health study. Am J Epidemiol. 2007;165:14–21. doi: 10.1093/aje/kwj346. [DOI] [PubMed] [Google Scholar]
- 9.Brouilette S, Singh RK, Thompson JR, Goodall AH, Samani NJ. White cell telomere length and risk of premature myocardial infarction. Arterioscler Thromb Vasc Biol. 2003;23:842–6. doi: 10.1161/01.ATV.0000067426.96344.32. [DOI] [PubMed] [Google Scholar]
- 10.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30:e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Physician's health study: aspirin and primary prevention of coronary heart disease. N Engl J Med. 1989;321:1825–8. doi: 10.1056/NEJM198912283212610. [DOI] [PubMed] [Google Scholar]
- 12.Samani NJ, Boultby R, Butler R, Thompson JR, Goodall AH. Telomere shortening in atherosclerosis. Lancet. 2001;358:472–3. doi: 10.1016/S0140-6736(01)05633-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.