FIGURE 4.
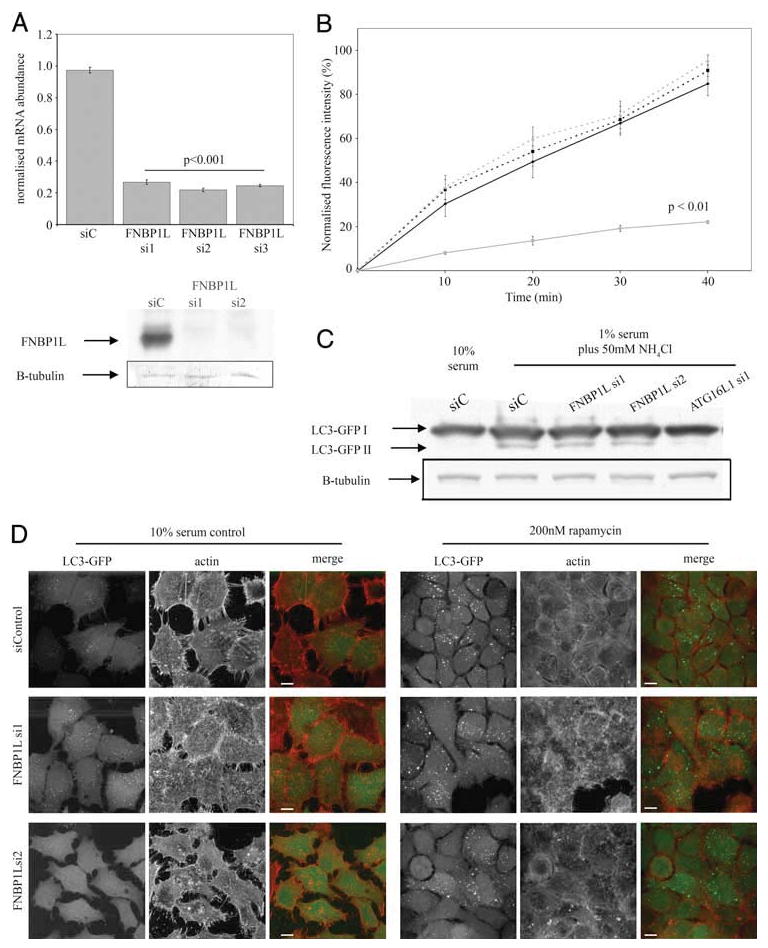
siRNA-mediated knockdown of FNBP1L has no effect upon transferrin endocytosis or autophagy induced by classical stimuli. A, Effective knockdown of FNBP1L mRNA in HeLa cells is achieved by all three siRNA duplexes (si1, si2, and si3) directed against FNBP1L, compared with a control (nontargeting) duplex. Mean FNBP1L mRNA abundance values, normalized to GAPDH control reactions, are shown (±SEM). The p values were calculated using a one-way ANOVA followed by a post hoc Student's t test using the Bonferroni correction compared to siControl (siC) values. Data were calculated from three separate samples for each condition, with PCR performed in duplicate. The lower panels show endogenous FNBP1L protein, detected using FNBP1L-specific Abs, in cells transfected with control and the FNBP1L si1 and si2 RNAi duplexes. The same blots, reprobed for β-tubulin (B-tubulin) are shown to confirm equal loading. B, Transferrin endocytosis is unaffected by FNBP1L knockdown. HeLa cells were transfected with control or FNBP1L-directed siRNAs or a dominant negative dynamin (K44A) construct (as a control for endocytosis inhibition) and 48 h later were serum-starved for 1 h and exposed to fluorescently labeled transferrin for up to 40 min. Following washing, cells were placed on ice and analyzed by FACS. Normalized average fluorescent intensities from triplicate samples are shown (±SEM). Statistical comparison by two-way ANOVA, followed by post hoc t tests (Bonferroni corrected) showed only dominant negative dynamin-expressing samples to be significantly different (p < 0.01 at all time points). C, FNBP1L knockdown does not inhibit classical autophagy induced by serum starvation and ammonium chloride treatment. HeLa LC3-GFP cells were transfected with control and FNBP1L-directed or ATG16L1-directed siRNAs and autophagy was induced after 48 h by incubation in 1% serum medium with 50 mM ammonium chloride for 4 h. Cells were then lysed, equalized for protein amounts, and blotted with anti-GFP Abs following SDS-PAGE. Autophagy was quantified by the generation of the lipidated LC3-GFP product (LC3-GFPII); note the absence of this product in 10% serum control-treated cells and the loss of product in ATG16L1-deficient cells (as previously published in Ref. 6). No differences in the amount of LC3-GFP II produced were observed between similarly treated control and FNBP1L-deficient cells. D, FNBP1L knockdown does not inhibit classical autophagy induced by rapamycin. HeLa LC3-GFP cells transfected with control or FNBP1L-directed siRNAs were either grown in 10% serum (left panels) or treated with 200 nM rapamycin for 24 h (right panels). No differences were observed in either case, with robust LC3-GFP (green in merge columns) vesicle and aggregate formation seen in all cases upon rapamycin treatment. The actin cytoskeleton of cells (red in merge columns) was also unaffected by siRNA treatments. Images are projections of confocal z-stacks; scale bars represent 10 μm.
