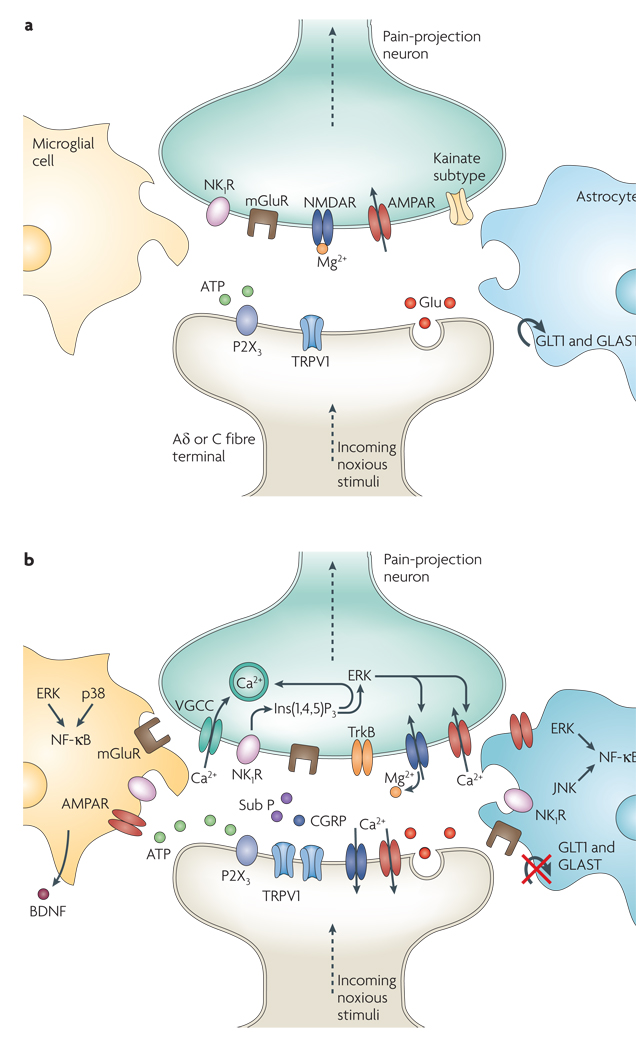
Figure 2. Molecules involved in pain processing.
a | Under healthy circumstances, low-frequency activation of Aδ and C fibre nociceptors by mild noxious stimuli leads to glutamate (Glu) release from the central presynaptic afferent nerve terminals in the spinal cord dorsal horn. Short-term activation of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid) and kainate subtypes of ionotropic glutamate receptors ensues. Although also present, the NMDA (N-methy-l-d-aspartate) ionotropic glutamate receptor subtype (NMDAR) remains silent because it is plugged by Mg2+. This signalling to dorsal horn pain-projection neurons provides information about the time of onset, duration and intensity of noxious stimuli from the periphery. Both astrocytes and microglia remain unchanged by these synaptic events. b | After repetitive synaptic communication, which can occur after a short barrage of nociceptive afferent input, there is an increase in the responsiveness of dorsal horn pain-projection neurons to subsequent stimuli (known as central sensitization) (see BOX 1). A co-release of glutamate and neurotransmitters such as substance P (sub P) and calcitonin gene-related peptide (CGRP) mediates NMDAR activation, leading to voltage-gated Ca2+ currents (VGCCs). In addition, inositol-1,4,5-trisphosphate (Ins(1,4,5)P3) signalling and mitogen-activated protein kinases, such as extracellular signal-regulated kinase (ERK), p38 and c-Jun N-terminal kinase (JNK), are activated. In neurons, ERK can further sensitize excited AMPA receptors (AMPARs) and NMDARs. Activation of purinoreceptors (P2X3) by ATP, activation of sub P receptors (the neurokinin 1 receptor (NK1R)), activation of metabotropic glutamate receptors (mGluR), and release of brain-derived neurotrophic factor (BDNF) all contribute to enhanced nociceptive transmission. Astrocytes and microglia express various neurotransmitter receptors and are activated by glutamate, ATP and sub P. At synapses the glutamate transporters, glutamate transporter 1 (GLT1) and glutamate–aspartate transporter (GLAST), which are crucial for clearing synaptic glutamate, become dysregulated after prolonged exposure to high levels of synaptic glutamate. Ongoing excitation can induce ERK, p38 and JNK activation in microglia and astrocytes. Each of these kinases can activate the transcription factor nuclear factor-κB (NF-κB), which induces the synthesis of inflammatory factors. Upregulation of the V1 transient receptor potential channel (TRPV1) after inflammation further contributes to the sensitization to noxious signals. During this time, normally non-nociceptive Aβ fibres can also activate pain-projection neurons.