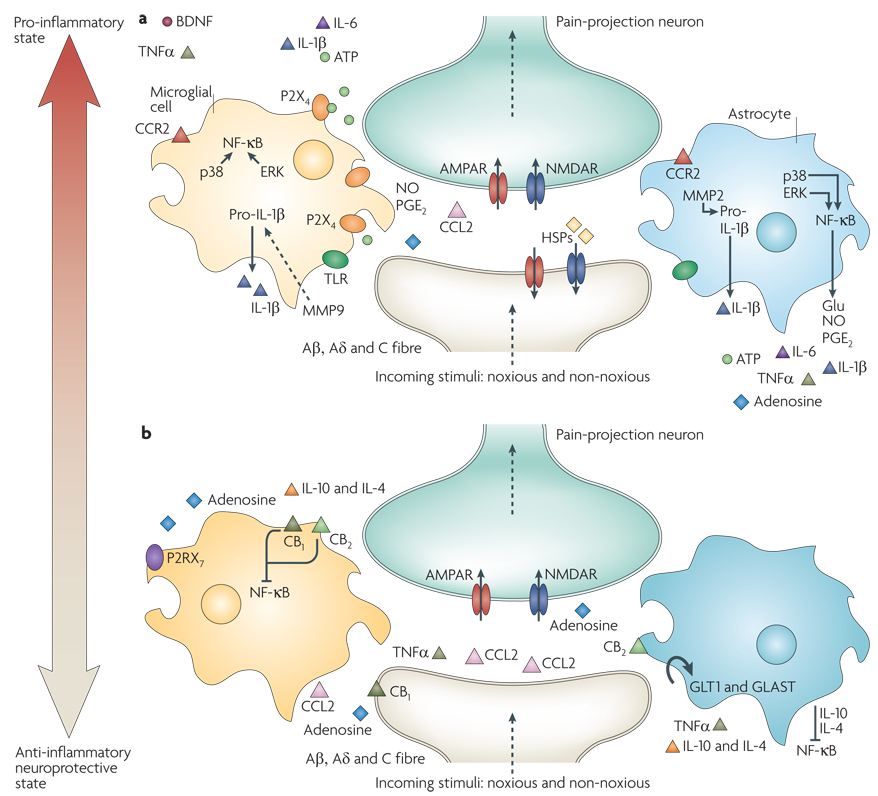
Figure 3. Glia activation: from pro-inflammatory to anti-inflammatory states.
a |Pro-inflammatory roles for glia. If a noxious input persists, such as during chronic inflammation or nerve damage, sustained central sensitization leads to transcriptional changes in dorsal horn neurons that alter these neurons’ function for prolonged periods. Astrocytes respond to this ongoing synaptic activity by mobilizing internal Ca2+, leading to the release of glutamate (Glu), ATP that binds to P2X4, tumour-necrosis factor-α (TNFα), interleukin 1β (IL-1 β), IL-6, nitric oxide (NO) and prostaglandin E2 (PGE2). Activated microglia are also a source of all of these pro-inflammatory factors. Matrix metalloproteinase 9 (MMP9) induces pro-IL-1β cleavage and microglial activation, whereas MMP2 induces pro-IL-1β cleavage and maintains astrocyte activation. The activation of p38 mitogen-activated protein kinase (p38 MAPK) is induced in both microglia and astrocytes on IL-1β signalling. Astrocytes and microglia express the chemokine receptors CX3CR1 (not shown) and CCR2 and become activated when the respective chemokines bind. After nerve damage, heat shock proteins (HSPs) are released and can bind to Toll-like receptors (TLRs) expressed on both astrocytes and microglia, leading to the further activation of these cell types. b | Activated glia can also be neuroprotective, as they release anti-inflammatory cytokines such as IL-10 and IL-4 and express cannabinoid receptors (CB1 and CB2) that have been shown to exert anti-inflammatory functions and to inhibit microglial toxicity by suppressing chemotactic responses and MAPK signal transduction, with consequent pro-inflammatory cytokine inhibition134,135. The glutamate transporters, glutamate transporter 1 (GLT1) and glutamate– aspartate transporter (GLAST), can resume normal glutamate clearance. Activation of microglial P2RX7 purinoreceptors by ATP leads to TNFα release that protects neurons from glutamate-induced toxicity. Activated astrocytes reduce the spread of tissue degeneration after direct injury through the controlled removal of dying neurons and tissue debris, another neuroprotective effect. In quiescent cells (not shown), nuclear factor-κB (NF-κκB) is sequestered in the cytosol by inhibitor of κb (IκB), which binds to specific regions on NF-κB and thereby prevents exposure of the nuclear-localization signal. On stimulation with pro-inflammatory cytokines, IκB proteins are phosphorylated, leading to their ubiquitin-dependent degradation. As a consequence, NF-κB translocates to the nucleus and binds to elements in the promoters of target genes, leading to activation of pro-inflammatory cytokine genes26