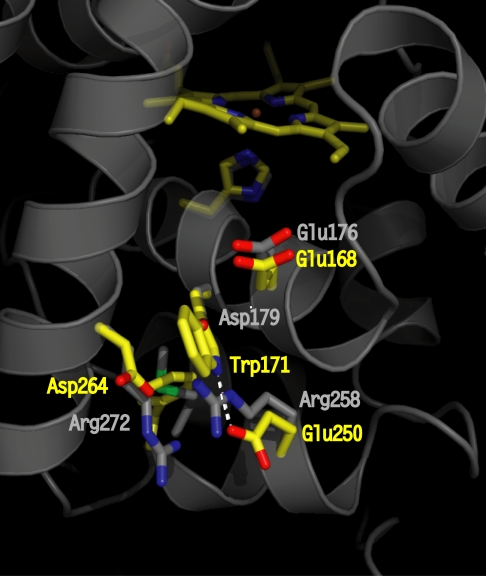
Fig. 1.
Crystallographic structure of LiP highlighting the site (residues in yellow) of the catalytically active Trp radical (Trp-171) and its acidic microenvironment (Glu-250, Glu-168, and Asp-264). The residues at homologous positions in WT CiP are shown in gray. The Asp-179 (in CiP) completely overlaps with Trp-171 (in LiP); thus, only the oxygen from the carboxylic group is visible. The figure was prepared by using the coordinates deposited in the Protein Data Bank (accession nos. 1B82 for LiP and 1LYC for CiP).