Figure 3. 13C NMR (I) and 1H NMR (II) spectra of [1′-13C]inosine, [1-13C]ribose and hypoxanthine.
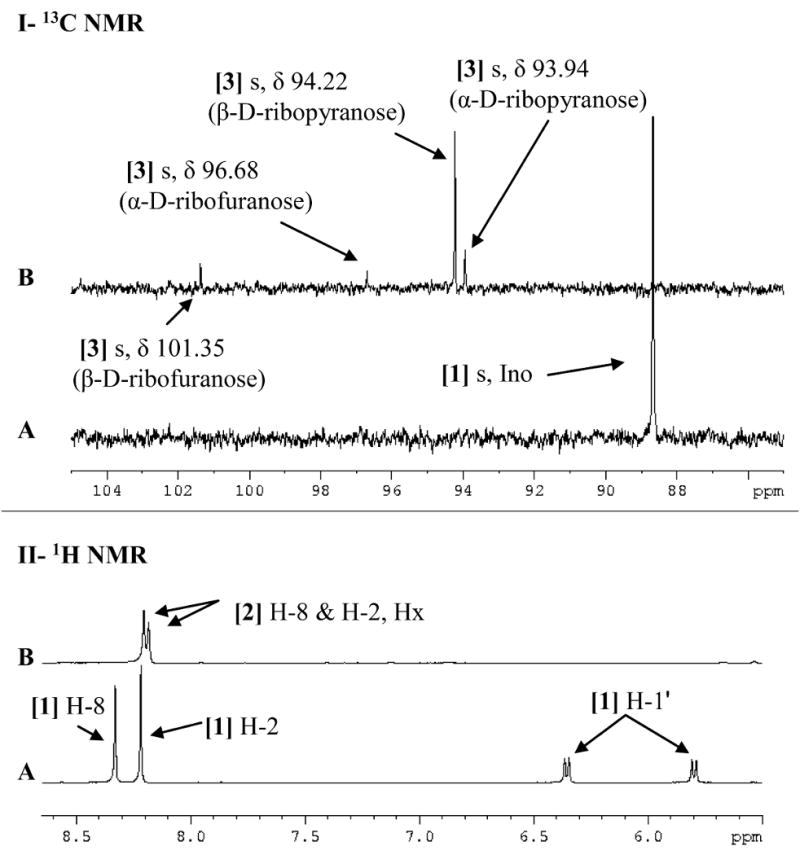
Spectra are of (A) 5.3 mM free [1′-13C]inosine and (B) [1-13C]ribose and hypoxanthine obtained through the irreversible arsenolysis of 5.3 mM [1′-13C]inosine in the presence of human PNP and 50 mM Na2HAsO4 (pH 7.4). Upfield signals have been omitted to highlight the downfield regions of interest. All spectra were acquired at 25 °C in 10% D2O. [1] Ino, inosine; [2] Hx, hypoxanthine.
