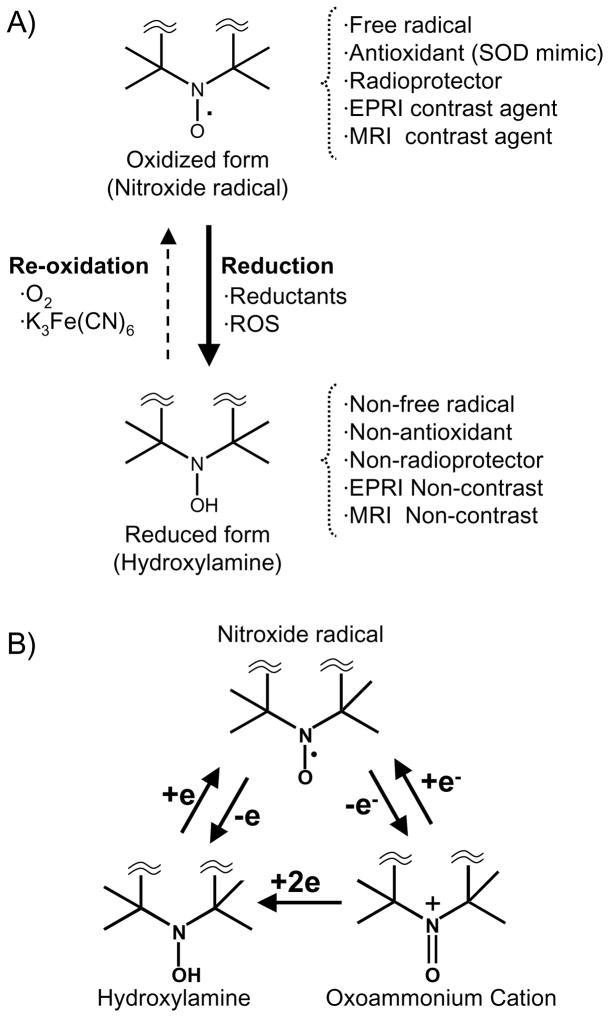
Figure 1.
A) Reversible one-electron reduction/oxidation showing the inter-conversion between the oxidized nitroxide (EPRI and MRI Contrast) and the corresponding reduced hydroxylamine (EPRI and MRI Non-contrast). B) Conversion of nitroxide radical to hydroxylamine or oxoammonium cation in vivo. Nitroxide compounds are found in vivo in an equilibrium between the nitroxide radical form which is detected by EPR, and the reduced form, known as the hydroxylamine, which is not detected. This equilibrium is dependent on the oxygen status and redox-status of the tissue milieu. Cellular redox processes convert the compound between the two states, thus the ratio of the two states is determined by the redox activity within the cell.