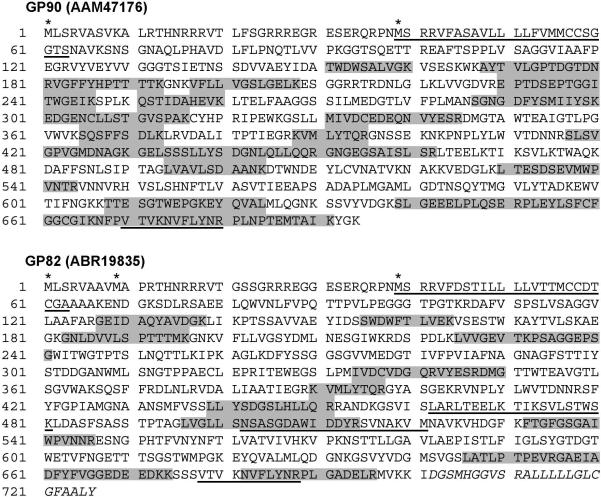
Figure 3.
Peptide-mapping coverage of GP90 and GP82. Representative fitting of tryptic peptides (shaded in gray) from 3 independent samples of GPI-rich extracts from metacyclic trypomastigotes that match with the deduced proteins GP90 (AAM47176) and GP82 (ABR19835) are shown. Potential initiator methionine residues are indicated by asterisks. Underlined sequences indicate the following motifs: signal-anchor or uncleaved signal peptide (positions 39−63 in GP90 and GP82); P4 and P8 cell-binding sites of GP82 (positions 462−481 and 502−521, respectively); FLY domain (positions 670−680 and 678−688 in GP90 and GP82, respectively). The hydrophobic C-terminal region of GP82, characteristic of GPI-anchored proteins, is indicated in italics (positions 702−726). The coverage percentage for GP90 and GP82 is 40% and 22%, respectively.