Abstract
Despite the increasing use of the rectal balloon in prostate cancer radiotherapy, many issues still remain to be verified objectively including its positional reproducibility and relevance to treatment morbidity. We have developed a custom rectal balloon that has a scale indicating the depth of insertion and dilates symmetrically ensuring positional reproducibility. Fifty patients with prostate cancer treated by definitive 3D-conformal radiotherapy (3D-CRT) or intensity-modulated radiotherapy (IMRT) with rectal balloon were analyzed. Each of first five patients undergone computed tomography (CT) three times with a rectal balloon. The positional reproducibility was tested by Intraclass Correlation Coefficient (ICC) from the CT-to-CT fusion images. Planning variables and clinical acute toxicities were compared between when or not applying balloon. An ICC of greater than 0.9 in all directions revealed an excellent reproducibility of the balloon. Rectal balloon improved considerably the mean dose and V45Gy-V65Gy in plan comparison, and especially in 3D-CRT the rectal volume exposed to more than 60 Gy dropped from 41.3% to 19.5%. Clinically, the balloon lowered acute toxicity, which was lowest when both the balloon and IMRT were applied simultaneously. The rectal balloon carries excellent reproducibility and reduces acute toxicity in 3D-CRT and IMRT for prostate cancer.
Keywords: Rectal Balloon; Prostatic Neoplasms; Radiotherapy, Intensity-Modulated; 3D Conformal Radiotherapy; Acute Toxicity
INTRODUCTION
The notion that 3D-conformal high dose (>75 Gy) radiation produces superior outcome to low dose radiation (<70 Gy) during prostate cancer radiotherapy has been revealed by several studies, in which the dose-response has been observed for a dose range of 63-81 Gy (1-3). More recently ultra-high dose intensity-modulated radiotherapy (IMRT) with dose escalation of even up to 86.4 Gy has been reported for localized prostate cancer (4). However, an important obstacle to high-dose high-precision radiotherapy for localized prostate cancer is that the target position changes depending on the state of the rectum and bladder. Many methods have been used in an attempt to minimize the inaccuracy that results from the positional shift of the prostate during prostate cancer radiotherapy. Such procedures include the daily localization method that uses a fiducial marker (5, 6) or B-mode acquisition and targeting (BAT) (Nomos Corporation, Sewickly, PA, U.S.A.) (7), and the rectal balloon (8-11) that immobilizes the prostate itself. Growing interest in the use of a rectal balloon for immobilizing the prostate led to our development of a unique rectal balloon that has been applied during prostate cancer radiotherapy in our institution since 2003.
The rectal balloon technique has two major advantages: it immobilizes the prostate and reduces treatment-related toxicity by saving the rectal wall (12-14). The rectal balloon restricts prostate motion by reducing both the inter- and intra-fractional movements. This minimizes the error caused by the motion of the prostate making it unnecessary to change the isocenter. The subsequent relative reduction in the rectal irradiation volume is expected to significantly reduce early and chronic side effects. To achieve both benefits effectively, reproducibility of the position and shape of the rectal balloon is required. Several studies have reported that the rectal balloon can reproduce the intra-pelvic position and reduce the planning target volume (PTV) margin (11, 12, 15, 16). However, other studies have reported that the movement of the anterior surface of the balloon remained high (17, 18). Several factors affect the intra-pelvic positioning of a rectal balloon including the air volume inside the balloon, the shape (symmetry) of the balloon, the insertion depth of the rectal tube, and bowel gas. To date, the effects of these factors have not been studied in depth.
Here, we have developed a unique rectal balloon that dilates symmetrically allowing high reproducibility in shape, has a scale that indicates the depth of insertion, and immobilizes not only the prostate but also the seminal vesicles due to the positioning of the tip of the rectal tube inside the balloon. There have been several reports on the positional reproducibility of rectal balloons but few have demonstrated positional reproducibility using a systematic and objective method. To our knowledge, the present study is the first to demonstrate the positional reproducibility of rectal balloons using systematic methods and reasonable statistical analysis. The positional reproducibility was tested by Intraclass Correlation Coefficient (ICC) from the computed tomography (CT)-to-CT fusion images. A comparative planning study verified the dosimetrical benefits of the rectal balloon by comparing the dose distribution between the cases with and without inserting the rectal balloon. And also we examined patient tolerance and toxicity of the rectal balloon in 50 patients treated using the balloon.
MATERIALS AND METHODS
Development of a highly reproducible rectal balloon
During the development of a rectal balloon, the positional and formative reproducibility, control of insertion depth, length of immobilization, and optimal volume of the rectal balloon have to be considered. For positional reproducibility, we used a balloon that allowed symmetric dilatation so that the rectal balloon could maintain high formative reproducibility during each treatment while still being pressured evenly by its surrounding structures. In addition, a scale was placed on the rectal tube to indicate the depth of insertion. The tip of the rectal tube was placed inside the balloon so that the balloon immobilized not only the upper part of the prostate but also the level of the seminal vesicles (Fig. 1, 2A). The basic form of the rectal balloon was a rectal tube used for enemas, with a plastic support inside the rectal tube for easy advancement once the tube was inserted. In addition, a 60 mL syringe was used to inflate the balloon and forceps were used to prevent air leaking. Before the balloon was inserted, it was capped with a latex condom and lubricating jelly was applied. In order to measure the depth of insertion of the balloon, graduations were marked on the rectal tube. To assess air leakage, we left a balloon inflated with 60 mL of air for seven days and confirmed that no air leaked from the balloon during that time.
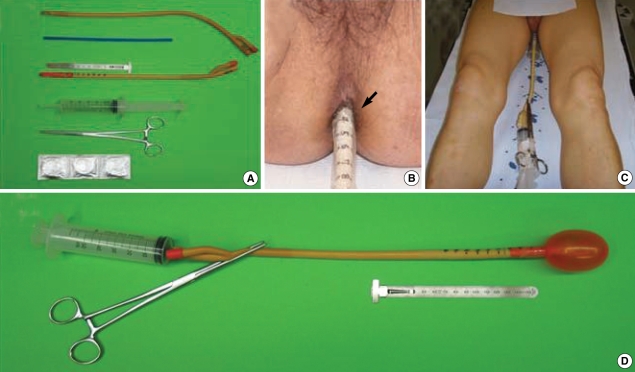
Fig. 1.
The rectal balloon and its application to the patient. (A) Constituents of the rectal balloon. (B) Measurement of the insertion depth of the rectal balloon using a marked scale on the rectal tube (black arrow). (C) Pre-treatment supine position. (D) Inflated rectal balloon.
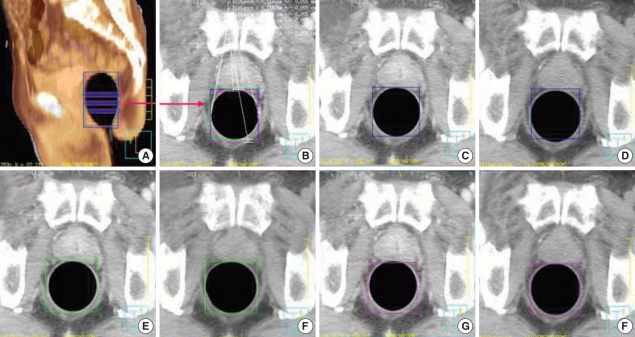
Fig. 2.
Positional reproducibility test for the rectal balloon. (A) Three levels of balloon displacement measurements on the fused sagittal image between the first (gray) and second CT (thermal). (B) Displacement measurement of the mid-rectangles between the first, second, and third CT. (C, D) Displacement of the balloon on the third CT compared to the first CT. (E, F) Displacement of the balloon on the first CT compared to the second CT. (G, H) Displacement of the balloon on the first CT compared to the third CT.
The rectal balloon was applied to patients as follows. First, a deflated rectal catheter was inserted gently into the patient with his knees bent. Then, the balloon was inflated slowly with 60 mL of air. Forceps were used to prevent air leakage. The graduations on the rectal balloon were recorded so that each insertion would be made at the same depth. Finally, the patient straightened his legs and radiotherapy was conducted in the supine posture.
Positional reproducibility of the rectal balloon
To prove the positional reproducibility of the rectal balloon, CT scans were performed 3 times in each of the first 5 patients with the rectal balloon. A total of 15 CT image sets were registered on the Philips treatment planning platform using the Pinnacle software and went through CT-to-CT fusion, in which the landmark was the pelvic bone. On each CT image set, the upper, middle, and lower sections passing through the prostate were selected. And the rectangles passing the edge of the rectal balloon on a total of 45 cross-sectional images were drawn on the radiotherapy planning system (RTPS). Then, we could get a coordinate value, which makes the variation of the x-axis and z-axis of the balloon edge measurable objectively from each line at 4 directions of the rectangles from the RTPS (Fig. 2). Through this procedure, 180 coordinates were obtained from 15 CT image sets. We defined 1 class as 15 coordinates from a level of the prostate and each direction of the rectangles, resulting in 12 classes. To determine that the coordinates representing the position of the rectal balloon reproduce with statistical significance for the classes, ICC was obtained (19, 20). We calculated how many patients we needed for statistically meaningful data of the reproducibility of CT taken 3 consecutive times from the same patient using the Power Analysis and Sample Size (PASS) program. The results showed that the number of patients suggested by the PASS program should be 5 or more at the 5% level of significance, a power of 80%, baseline ICC r0=0.50, and expected ICC r1=0.90 or higher. During actual treatment, the position of the rectal balloon was monitored weekly by port film or electronic-portal imaging device (Fig. 3).
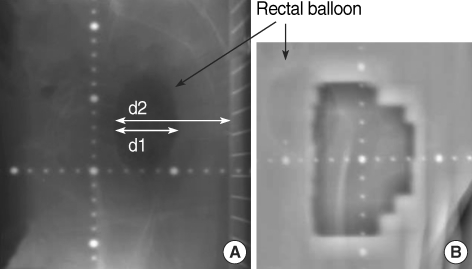
Fig. 3.
Identification of the rectal balloon (blackarrow) on: (A) the verification film (RIT 113®) and (B) the EPID image on a lateral beam port. d1 represents the sagittal diameter of the balloon, d2 represents the distance between the anterior surface of the balloon and the top of the couch table.
Radiation treatment
For the bladder, CT-simulation or treatment was performed in the supine posture after the bladder was filled for a specific period of time within a comfortable range for the patient. Elective pelvic lymph node irradiation was performed only when the possibility of pelvic lymph node metastasis estimated by the Roach score was greater than 30%.
3D-CRT was planned using the ADAC Pinnacle3® system, and the fraction dose was 1.8 Gy. Pelvic lymph nodes and distal seminal vesicles were excluded through the first cone down (CD) at 45.0-50.4 Gy, and proximal seminal vesicle and periprostatic fat were excluded through the second CD at 59.4 Gy. The prostate gland and gross tumor volume (GTV) were irradiated up to 70.2 Gy. IMRT was applied using the simultaneous integrated boost (SIB) technique, in which the target volumes of three layers (high-dose PTV (HPTV), mid-dose PTV (MPTV) and low-dose PTV (LPTV) were contoured resulting in a different fractional dose for each target. HPTV included the prostate gland and any extraprostatic gross tumor, MPTV included the periprostatic fat tissue and proximal seminal vesicles, and LPTV included the distal seminal vesicles, set-up errors and pelvic lymph nodes. Irradiation was performed with a total of 28 fractions. The HPTV received a total dose of 70 Gy at 2.5 Gy per fraction, the MPTV received a total dose of 60.2 Gy at 2.15 Gy per fraction, and the LPTV received a total dose of 50.4 Gy at 1.8 Gy per fraction. In patients with a Roach score of 30 or less, the pelvic lymph node chain was excluded from the LPTV. IMRT plans were calculated using the Corvus inverse planning system (Nomos Corporation), and step and shoot IMRT was carried out using PRIMUS® (Siemens Medical Solution, Erlangen, Germany).
For patients treated by 3D-CRT in this study, the rectal balloon was not applied to the first treatment but cone down because it was thought that 39 fractions could be a burden to both radiotherapy technicians and patients of the use of rectal balloons. Nowadays, since the procedure takes just 1 or 2 min with the proper training, most 3D-CRT patients feel comfortable with the insertion of rectal balloons from the first treatment. In IMRT patients in this study, we applied the rectal balloon from the beginning since the period of treatment was reduced by more than 2 weeks with the hypofractionated regimen. Posterior shielding or rectal block was not performed throughout the treatment.
Comparative planning study between cases treated with and without the rectal balloon
We chose 10 patients in whom both CT images with and without insertion of the balloon were available and conducted 3D-CRT and IMRT for each patient and compared the distribution of rectal doses between the cases. Target volumes were prostate only in 5 patients and whole pelvis in the others. Dose statistics such as mean rectal dose, V45Gy-V65Gy (VnGy, volume receiving n Gy) and dose volume histogram (DVH) were compared between plans from each treatment method with or without the rectal balloon. For the 3D-CRT plan with the rectal balloon, we assumed that the balloon was applied from the beginning according to the current treatment scheme. Non-parametric Wilcoxon rank sum test was performed to prove statistical significance between the plans with and without the balloons in each treatment. In addition, the 3D-CRT plan with the balloon was compared to the IMRT plan with no balloon.
Evaluation of acute toxicity and patient tolerance
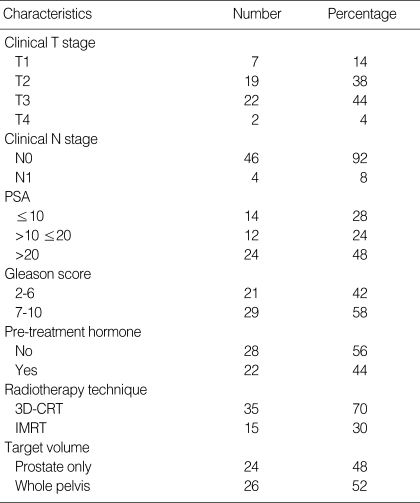
Fifty prostate cancer patients who had received radical radiotherapy (3D-CRT 35, IMRT 15 patients) between February 2004 and June 2006 were evaluated for acute toxicity and patient tolerance. Table 1 shows the details of the patients and their treatment. Examination was made once a week during the period of treatment, once a month for three months after treatment, once every three months for a year after treatment, and afterward once every six months. Toxicity related to treatment was assessed at each visit and acute toxicity was measured using the Radiation Therapy Oncology Group (RTOG) toxicity score (21). Acute toxicity was defined as the side effects related to radiotherapy that occurred during treatment and within three months after the completion of treatment.
Table 1.
Patient characteristics
PSA, prostate-specific antigen; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy.
RESULTS
Excellent positional reproducibility of the rectal balloon
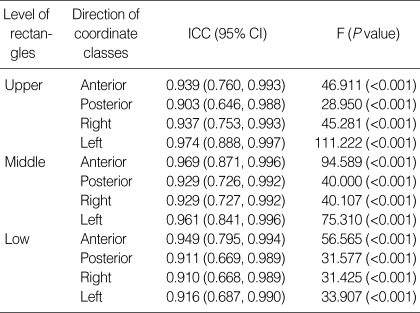
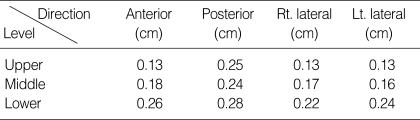
Excellent positional reproducibility of the rectal balloon was proved objectively from ICC's greater than 0.90 in all directions. The anterior line of the balloon, which represents the interface between the rectum and prostate, showed an ICC of 0.94 and above in all of the upper, middle, and lower levels, giving us high reliability for prostate immobilization (Table 2). The standard deviation (SD) of the positional displacement ranged from 1.3 mm to 2.8 mm, demonstrating an accuracy of less than 3 mm (Table 2, 3). Among them, every directions of the low rectangle had a SD larger than 2 mm, which might be attributed to the small balloon volume in the lower part of the rectum closer to the anal sphincter. Interestingly, the SD of the anterior line, where the prostate meets the anterior rectal wall, was smaller than that of the posterior line in both the upper and middle rectangles. The excellent reproducibility was mainly due to the creative characteristics of our rectal balloon, including the symmetry of rectal balloon dilatation, a scale indicating the depth of insertion, and the ability of the balloon to immobilize the seminal vesicle as well as the prostate due to the positioning of the tip of the rectal tube inside the balloon.
Table 2.
Intraclass correlation coefficient for positional reproducibility of rectal balloon
CT, computed tomography; ICC, intraclass correlation coefficient; 95% CI, 95% confidence interval.
Table 3.
Standard deviations of each direction of rectal balloon
Rectal balloon technique dosimetry: superiority of the rectal balloon in terms of rectal wall saving
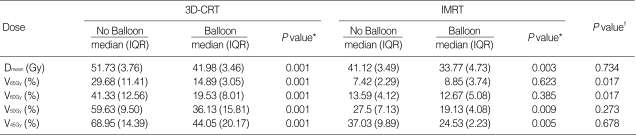
The rectal balloon improved rectal wall saving compared to patients who did not receive rectal balloon treatment (Table 4, Fig. 4). In detail the benefit of rectal wall sparing using the rectal balloon was more remarkable in patients receiving 3D-CRT than IMRT.
Table 4.
Rectal dose statistics according to radiotherapy technique and use of balloon
*Comparison between no balloon and balloon of 3D-CRT/IMRT with Wilcoxon rank sum test; †Comparison between no balloon of IMRT and balloon of 3D-CRT with Wilcoxon rank sum test.
The rectal percent volumes receiving 65 Gy, 60 Gy, 50 Gy, and 45 Gy are presented as V65Gy, V60Gy, V50Gy, and V45Gy, respectively.
3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; IQR, InterQuartile Range; Dmean, mean dose.
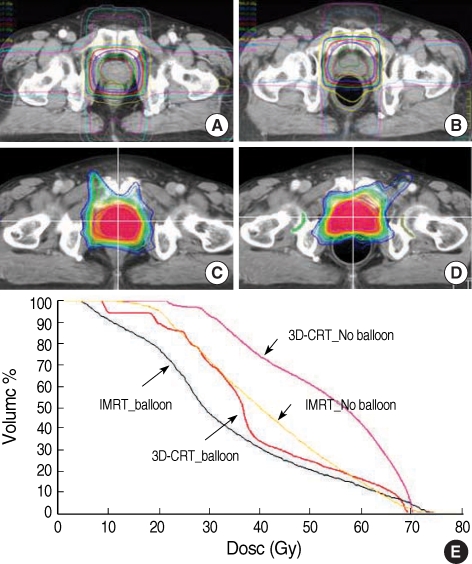
Fig. 4.
Change in dose distribution and dose volume histogram (DVH) according to the balloon in prostate cancer radiotherapy. (A) 3D-CRT without balloon. (B) 3D-CRT with balloon. (C) IMRT without balloon. (D) IMRT with balloon. (E) Rectal DVH.
In the 3D-CRT plan, use of the rectal balloon resulted in a dose-reduction effect of more than 15 Gy in the mean rectal dose and decreased significantly all the values of V65Gy, V60Gy, V50Gy and V45Gy up to 15-20%. In the IMRT plan, the mean rectal dose was reduced from 41.1 Gy to 33.8 Gy through the use of the rectal balloon but in IMRT, it did not exceed 15% regardless of whether the balloon was used or not. In some of the high-dose ranges greater than 65 Gy, the rectal volume increased slightly during IMRT when the balloon was used, probably due to the effects of the balloon pushing against the rectal wall. However, this increase was so small that it was not clinically relevant. V50Gy and V45Gy in IMRT decreased significantly with the balloon.
When we compared the plans between no balloon IMRT and balloon 3D-CRT, there were no significant differences in the mean rectal dose, V50Gy and V45Gy, implying that use of rectal balloon in 3D-CRT could produce favorable dosimetry effect comparable to that of IMRT without balloon.
Acute toxicity and patient tolerance in the definitive radiotherapy using rectal balloon for prostate cancer
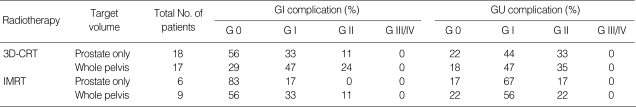
Since many of the patients in this study had an initial prostate-specific antigen (PSA) of over 20 and an advanced prostate cancer of over T3 and GS7, in many cases the target volume included the pelvic lymph nodes. Inclusion of the pelvic lymph nodes within the target volume is an important variable not only in the plan comparison but also with respect to the gastrointestinal (GI) complication rate. Thus, we examined the occurrence of acute complications in the patients with respect to the radiotherapy technique applied and the target volume (Table 5). In addition, when determining whether to use the rectal balloon, we compared our results with recent reports (8, 10, 22-27) describing the complications that result from 3D-CRT and IMRT (Table 6). Based on these previous studies, fewer rectal complications were observed when a rectal balloon was used and IMRT was applied. In this study, we also found that in patients receiving prostate only IMRT, Grade I complications were detected in 17% (1/6) of patients whereas Grade II complications were not observed. In cases of whole pelvis IMRT, the percentage of Grade I complications increased to 33%, but again Grade II complications were relatively rare. In patients receiving 3D-CRT with the rectal balloon, side effects were rarely observed when radiotherapy was applied to the prostate only. However, when the pelvic lymph nodes were included in 3D-CRT, the reduction of side effects by the rectal balloon was insignificant.
Table 5.
Analysis of acute complications according to target volume and technique (RTOG Grade)
RTOG, Radiation Therapy Oncology Group; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; GI, gastrointestinal; GU, genitourinary.
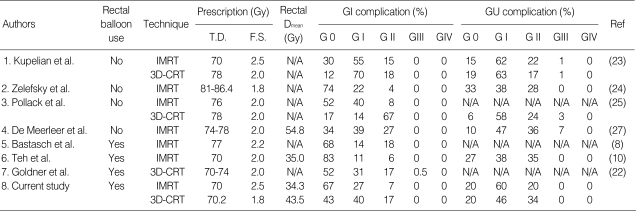
Table 6.
Acute complications following 3D-CRT & IMRT for prostate cancers in patients with and without balloon
3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; GI, gastrointestinal; GU, genitourinary; Gy, gray; T.D., total prescribed dose; F.S., fraction size; Dmean, mean dose; Ref, reference; N/A, not available.
None of the patients who had started treatment with the rectal balloon stopped treatment due to toxicity-related complications. During the period of study, one patient refused the insertion of the balloon from the beginning because existing hemorrhoids were too painful. Three patients that had started radiotherapy, later complained of pain during insertion of the rectal balloon due to worsening hemorrhoids, but all of them finished the treatment with the rectal balloon using oral analgesics. Most of the patients without hemorrhoids reported that the rectal balloon was tolerable throughout the entire period of treatment. The volume of air in the balloon was set at 60 mL for all of the patients.
DISCUSSION
The use of the rectal balloon during prostate cancer radiotherapy is increasing, but many issues still remain to be verified objectively including the positional reproducibility, optimal shape and volume, insertion depth control and relevance to clinical data. We have developed a uniquely designed rectal balloon that could achieve high reproducibility, immobilization effects not only for the prostate but also for the level of the seminal vesicles, and controls the insertion depth of the rectal tube. This study demonstrated the positional reproducibility of this rectal balloon using systematic methods and reasonable statistical analysis. We then verified the benefit of using the rectal balloon with respect to dosimetrical and clinical aspects through a comparative planning study and analysis of acute toxicities in patients treated with 3D-CRT and IMRT using the rectal balloon. Thus, the results and figures from this study provide meaningful information concerning the clinical use of the rectal balloon.
Many factors influence the intra-pelvic positioning of a rectal balloon including the air volume inside the balloon, the shape (symmetry) of the rectal balloon, the insertion depth of the rectal tube, and bowel gas. Of these factors, only the adequate air volume of the rectal balloon has been studied previously. The volume of a rectal balloon varies between 40-120 mL (8, 11-13, 15-18, 22, 28-30). Hille et al. (30) have reported that 60 mL allows increased reproducibility compared with 40 mL. With respect to rectal wall saving, it is considered desirable to inflate the balloon as much as is tolerable to the patient. Our institution applied 60 mL uniformly to all patients.
Another factor affecting the positioning of a rectal balloon is the shape of the balloon. Some reports have provided photographs of the rectal balloons actually used during therapy (11-13, 17, 22). Based on these reports, we have divided the shape of the rectal balloons into three subtypes according to the symmetry of balloon expansion and the exposure of the rectal tube tips. These groups include symmetric ballooning and the rectal tube tip remaining inside the balloon (11); symmetric ballooning and the rectal tube tip exposed at the top of the balloon (12, 13, 22); and asymmetric dilatation (17). In our case, the rectal tube tip was inside the balloon and the balloon expanded symmetrically, similar to that described by Watcher et al. (11). While exposure of the rectal tube tip at the top of the rectal balloon restricts cranial distension, the tip inside the balloon is advantageous in that the balloon can immobilize not only the prostate but also the seminal vesicles. In addition, symmetric dilatation provided by our balloon seems better for minimizing the effect of torsion when inserting the rectal tube and for distributing pressure evenly throughout the pelvis. One study describing the large movement of the anterior surface of the rectal balloon uses an asymmetric-shaped rectal balloon, which may account for the significant amount of movement (17).
The insertion depth of the rectal tube should be consistent. Generally, the tube is inserted fully before the balloon is inflated, and then the tube is removed until the dilated balloon is gently lodged on the anal sphincter. However, the position may differ depending on the patient's tension. El-Bassiouni et al. (18) reported that the craniocaudal directional variation was as large as 6.4 mm (±2.5 mm SD). However, this may be controlled to some degree by scaling the rectal tube (Fig. 1B). Scaling itself is very easily applicable to any rectal balloon.
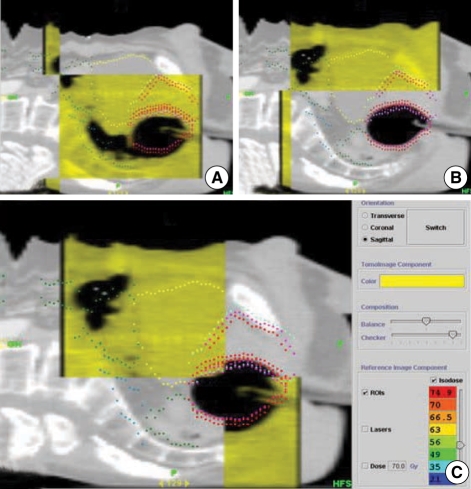
The last important factor affecting the positioning of rectal balloons is rectal gas. Although it does not happen frequently, rectal gas can change the position of the rectal wall and the prostate by pushing the rectal balloon. In the daily megavoltage CT (MVCT) of helical tomotherapy introduced recently to our institution, we observed that rectal gas causes a relatively large deviation in the positioning of rectal balloons (Fig. 5). Since the gas does not disappear immediately, in cases where the deviation is large, we would recommend deferring the treatment for several hours or until the following day. As there are various factors that affect the intra-pelvic positional variation of rectal balloons, it is considered meaningful to combine image-guided radiotherapy (IGRT) such as BAT and MVCT with rectal balloon treatment.
Fig. 5.
Balloon displacement by rectal gas identified on the megavoltage CT (MVCT) scan of helical tomotherapy. (A) Rectal gas and balloon on MVCT image (yellow). (B) Balloon on the initial CT image (gray). (C) Unmatched balloon surface between the MVCT and initial CT image.
The rectal balloon technique not only immobilized the prostate, but also reduced treatment-related toxicity through relative rectal wall saving. The relative reduction of rectal irradiation volume is expected to reduce early and chronic side effects significantly. In general, side effects from radiotherapy depend on the dose applied to specific organs and the volume percentage. In cases like hollow viscus that has radial distensibility, irradiation of the whole circumference would lead to stricture and an increased likelihood of bleeding when substances pass through. However, if only a small part of hollow viscus receives high-dose irradiation, then the remainder maintains radial distensibility. Subsequently, pressure from passing substances will be low, and obstruction and bleeding will be less likely. The rectal balloon has a tendency to push the anterior rectal wall toward the prostate. However, since the rectum and the prostate almost touch each other in most cases, an overlapping margin for the PTV is inevitable. In contrast, the expansion of the whole rectal wall by the balloon reduces the volume of the rectum exposed to high-dose radiation, which in turn reduces the side effects. The build-up effect caused by air inside the balloon may also contribute partially to decreased side effects (31).
Besides irradiation volume, acute side effects from prostate cancer radiotherapy can be affected by various factors (27) including planning variables (i.e., whether to include margin, seminal vesicles, pelvic lymph nodes), differences in toxicity scoring scales, and auto-medication as an over-the-counter drug. Accordingly, these factors need to be considered together when analyzing acute side effects. In our research, a relatively large number of patients had locally advanced prostate cancers, so pelvic lymph nodes were included in the target volume in many patients. Furthermore, during 3D-CRT, the rectal balloon was applied from 45-50 Gy considering the long treatment period of conventional fractionation. Therefore, we assessed patient tolerance and acute toxicity depending on the technique and target volume. We found that patients were able to tolerate the rectal balloon technique very well. Side effects were least when the rectal balloon was used in IMRT, although they were also quite rare when 3D-CRT was applied to the prostate only.
Recently, hypofractionated radiotherapy is widely performed due to a low α/β ratio in prostate cancers (32-35). The study sample used here also included patients treated by hypofractionated IMRT schedule delivering 70 Gy at 2.5 Gy/fraction in 5.5 weeks. In these cases, the reduction in the treatment period makes it easier to use rectal balloons. Considering the low α/β ratio in prostate cancers, hypofractionation is expected to increase the tumor control probability considerably. However, the increasing effect on geometric uncertainties can reduce tumor control probability (36). Thus, the benefit of hypofractionation can be maximized through minimizing geometric uncertainties using the rectal balloon. In addition, the shortening of treatment period would be expected to increase the practicality of using this technique.
In conclusion, the present study demonstrates the positional reproducibility of rectal balloons using systematic methods and reasonable statistical techniques. In addition, we showed that the rectal balloon reduces acute side effects consistently with the result of a dosimetrically outstanding plan.
References
- 1.Hanks GE, Hanlon AL, Schultheiss TE, Pinover WH, Movsas B, Epstein BE, Hunt MA. Dose escalation with 3D conformal treatment: five year outcomes, treatment optimization, and future directions. Int J Radiat Oncol Biol Phys. 1998;41:501–510. doi: 10.1016/s0360-3016(98)00089-3. [DOI] [PubMed] [Google Scholar]
- 2.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Uzzo RG, Hanks GE. Prostate cancer radiotherapy dose response: an update of the fox chase experience. J Urol. 2004;171:1132–1136. doi: 10.1097/01.ju.0000111844.95024.74. [DOI] [PubMed] [Google Scholar]
- 3.Zelefsky MJ, Leibel SA, Gaudin PB, Kutcher GJ, Fleshner NE, Venkatramen ES, Reuter VE, Fair WR, Ling CC, Fuks Z. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998;41:491–500. doi: 10.1016/s0360-3016(98)00091-1. [DOI] [PubMed] [Google Scholar]
- 4.Cahlon O, Zelefsky MJ, Shippy A, Chan H, Fuks Z, Yamada Y, Hunt M, Greenstein S, Amols H. Ultra-high dose (86.4 Gy) IMRT for localized prostate cancer: toxicity and biochemical outcomes. Int J Radiat Oncol Biol Phys. 2008;71:330–337. doi: 10.1016/j.ijrobp.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Vigneault E, Pouliot J, Laverdiere J, Roy J, Dorion M. Electronic portal imaging device detection of radioopaque markers for the evaluation of prostate position during megavoltage irradiation: a clinical study. Int J Radiat Oncol Biol Phys. 1997;37:205–212. doi: 10.1016/s0360-3016(96)00341-0. [DOI] [PubMed] [Google Scholar]
- 6.Balter JM, Lam KL, Sandler HM, Littles JF, Bree RL, Ten Haken RK. Automated localization of the prostate at the time of treatment using implanted radiopaque markers: technical feasibility. Int J Radiat Oncol Biol Phys. 1995;33:1281–1286. doi: 10.1016/0360-3016(95)02083-7. [DOI] [PubMed] [Google Scholar]
- 7.Lattanzi J, McNeeley S, Pinover W, Horwitz E, Das I, Schultheiss TE, Hanks GE. A comparison of daily CT localization to a daily ultrasound-based system in prostate cancer. Int J Radiat Oncol Biol Phys. 1999;43:719–725. doi: 10.1016/s0360-3016(98)00496-9. [DOI] [PubMed] [Google Scholar]
- 8.Bastasch MD, Teh BS, Mai WY, McGary JE, Grant WH, 3rd, Butler EB. Tolerance of endorectal balloon in 396 patients treated with intensity-modulated radiation therapy (IMRT) for prostate cancer. Am J Clin Oncol. 2006;29:8–11. doi: 10.1097/01.coc.0000195099.26957.63. [DOI] [PubMed] [Google Scholar]
- 9.D'Amico AV, Manola J, McMahon E, Loffredo M, Lopes L, Ching J, Albert M, Hurwitz M, Suh WW, Vivenzio TA, Beard C. A prospective evaluation of rectal bleeding after dose-escalated three-dimensional conformal radiation therapy using an intrarectal balloon for prostate gland localization and immobilization. Urology. 2006;67:780–784. doi: 10.1016/j.urology.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 10.Teh BS, Mai WY, Uhl BM, Augspurger ME, Grant WH, 3rd, Lu HH, Woo SY, Carpenter LS, Chiu JK, Butler EB. Intensity-modulated radiation therapy (IMRT) for prostate cancer with the use of a rectal balloon for prostate immobilization: acute toxicity and dose-volume analysis. Int J Radiat Oncol Biol Phys. 2001;49:705–712. doi: 10.1016/s0360-3016(00)01428-0. [DOI] [PubMed] [Google Scholar]
- 11.Wachter S, Gerstner N, Dorner D, Goldner G, Colotto A, Wambersie A, Potter R. The influence of a rectal balloon tube as internal immobilization device on variations of volumes and dose-volume histograms during treatment course of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52:91–100. doi: 10.1016/s0360-3016(01)01821-1. [DOI] [PubMed] [Google Scholar]
- 12.Teh BS, McGary JE, Dong L, Mai WY, Carpenter LS, Lu HH, Chiu JK, Woo SY, Grant WH, Butler EB. The use of rectal balloon during the delivery of intensity modulated radiotherapy (IMRT) for prostate cancer: more than just a prostate gland immobilization device? Cancer J. 2002;8:476–483. doi: 10.1097/00130404-200211000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Patel RR, Orton N, Tome WA, Chappell R, Ritter MA. Rectal dose sparing with a balloon catheter and ultrasound localization in conformal radiation therapy for prostate cancer. Radiother Oncol. 2003;67:285–294. doi: 10.1016/s0167-8140(03)00056-2. [DOI] [PubMed] [Google Scholar]
- 14.van Lin EN, Kristinsson J, Philippens ME, de Jong DJ, van der Vight LP, Kaanders JH, Leer JW, Visser AG. Reduced late rectal mucosal changes after prostate three-dimensional conformal radiotherapy with endorectal balloon as observed in repeated endoscopy. Int J Radiat Oncol Biol Phys. 2007;67:799–811. doi: 10.1016/j.ijrobp.2006.09.034. [DOI] [PubMed] [Google Scholar]
- 15.Court LE, D'Amico AV, Kadam D, Cormack R. Motion and shape change when using an endorectal balloon during prostate radiation therapy. Radiother Oncol. 2006;81:184–189. doi: 10.1016/j.radonc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 16.Ciernik IF, Baumert BG, Egli P, Glanzmann C, Lutolf UM. On-line correction of beam portals in the treatment of prostate cancer using an endorectal balloon device. Radiother Oncol. 2002;65:39–45. doi: 10.1016/s0167-8140(02)00187-1. [DOI] [PubMed] [Google Scholar]
- 17.van Lin EN, van der Vight LP, Witjes JA, Huisman HJ, Leer JW, Visser AG. The effect of an endorectal balloon and off-line correction on the interfraction systematic and random prostate position variations: a comparative study. Int J Radiat Oncol Biol Phys. 2005;61:278–288. doi: 10.1016/j.ijrobp.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 18.El-Bassiouni M, Davis JB, El-Attar I, Studer GM, Lutolf UM, Ciernik IF. Target motion variability and on-line positioning accuracy during external-beam radiation therapy of prostate cancer with an endorectal balloon device. Strahlenther Onkol. 2006;182:531–536. doi: 10.1007/s00066-006-1581-1. [DOI] [PubMed] [Google Scholar]
- 19.Munro BH. Statistical methods for health care research. 5th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. [Google Scholar]
- 20.Walter SD, Eliasziw M, Donner A. Sample size and optimal designs for reliability studies. Stat Med. 1998;17:101–110. doi: 10.1002/(sici)1097-0258(19980115)17:1<101::aid-sim727>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 21.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 22.Goldner G, Geinitz H, Wachter S, Becker G, Zimmermann F, Wachter-Gerstner N, Glocker S, Potzi R, Wambersie A, Bamberg M, Molls M, Feldmann H, Potter R. 3-D Conformal radiotherapy of localized prostate cancer within an Austrian-German multicenter trial: a prospective study of patients' acceptance of the rectal balloon during treatment. Wien Klin Wochenschr. 2006;118:224–229. doi: 10.1007/s00508-006-0588-z. [DOI] [PubMed] [Google Scholar]
- 23.Kupelian PA, Reddy CA, Carlson TP, Altsman KA, Willoughby TR. Preliminary observations on biochemical relapse-free survival rates after short-course intensity-modulated radiotherapy (70 Gy at 2.5 Gy/fraction) for localized prostate cancer. Int J Radiat Oncol Biol Phys. 2002;53:904–912. doi: 10.1016/s0360-3016(02)02836-5. [DOI] [PubMed] [Google Scholar]
- 24.Zelefsky MJ, Fuks Z, Hunt M, Yamada Y, Marion C, Ling CC, Amols H, Venkatraman ES, Leibel SA. High-dose intensity modulated radiation therapy for prostate cancer: early toxicity and biochemical outcome in 772 patients. Int J Radiat Oncol Biol Phys. 2002;53:1111–1116. doi: 10.1016/s0360-3016(02)02857-2. [DOI] [PubMed] [Google Scholar]
- 25.Pollack A, Zagars GK, Starkschall G, Childress CH, Kopplin S, Boyer AL, Rosen II. Conventional vs. conformal radiotherapy for prostate cancer: preliminary results of dosimetry and acute toxicity. Int J Radiat Oncol Biol Phys. 1996;34:555–564. doi: 10.1016/0360-3016(95)02103-5. [DOI] [PubMed] [Google Scholar]
- 26.Pollack A, Hanlon AL, Horwitz EM, Feigenberg SJ, Konski AA, Movsas B, Greenberg RE, Uzzo RG, Ma CM, McNeeley SW, Buyyounouski MK, Price RA., Jr Dosimetry and preliminary acute toxicity in the first 100 men treated for prostate cancer on a randomized hypofractionation dose escalation trial. Int J Radiat Oncol Biol Phys. 2006;64:518–526. doi: 10.1016/j.ijrobp.2005.07.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Meerleer G, Vakaet L, Meersschout S, Villeirs G, Verbaeys A, Oosterlinck W, De Neve W. Intensity-modulated radiotherapy as primary treatment for prostate cancer: acute toxicity in 114 patients. Int J Radiat Oncol Biol Phys. 2004;60:777–787. doi: 10.1016/j.ijrobp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 28.Sanghani MV, Ching J, Schultz D, Cormack R, Loffredo M, McMahon E, Beard C, D'Amico AV. Impact on rectal dose from the use of a prostate immobilization and rectal localization device for patients receiving dose escalated 3D conformal radiation therapy. Urol Oncol. 2004;22:165–168. doi: 10.1016/j.urolonc.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Ronson BB, Yonemoto LT, Rossi CJ, Slater JM, Slater JD. Patient tolerance of rectal balloons in conformal radiation treatment of prostate cancer. Int J Radiat Oncol Biol Phys. 2006;64:1367–1370. doi: 10.1016/j.ijrobp.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Hille A, Schmidberger H, Tows N, Weiss E, Vorwerk H, Hess CF. The impact of varying volumes in rectal balloons on rectal dose sparing in conformal radiation therapy of prostate cancer. A prospective three-dimensional analysis. Strahlenther Onkol. 2005;181:709–716. doi: 10.1007/s00066-005-1443-2. [DOI] [PubMed] [Google Scholar]
- 31.Teh BS, Dong L, McGary JE, Mai WY, Grant W, 3rd, Butler EB. Rectal wall sparing by dosimetric effect of rectal balloon used during intensity-modulated radiation therapy (IMRT) for prostate cancer. Med Dosim. 2005;30:25–30. doi: 10.1016/j.meddos.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 32.Kupelian PA, Thakkar VV, Khuntia D, Reddy CA, Klein EA, Mahadevan A. Hypofractionated intensity-modulated radiotherapy (70 gy at 2.5 Gy per fraction) for localized prostate cancer: long-term outcomes. Int J Radiat Oncol Biol Phys. 2005;63:1463–1468. doi: 10.1016/j.ijrobp.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 33.Livsey JE, Cowan RA, Wylie JP, Swindell R, Read G, Khoo VS, Logue JP. Hypofractionated conformal radiotherapy in carcinoma of the prostate: five-year outcome analysis. Int J Radiat Oncol Biol Phys. 2003;57:1254–1259. doi: 10.1016/s0360-3016(03)00752-1. [DOI] [PubMed] [Google Scholar]
- 34.Brenner DJ, Hall EJ. Fractionation and protraction for radiotherapy of prostate carcinoma. Int J Radiat Oncol Biol Phys. 1999;43:1095–1101. doi: 10.1016/s0360-3016(98)00438-6. [DOI] [PubMed] [Google Scholar]
- 35.Fowler JF, Ritter MA, Chappell RJ, Brenner DJ. What hypofractionated protocols should be tested for prostate cancer? Int J Radiat Oncol Biol Phys. 2003;56:1093–1104. doi: 10.1016/s0360-3016(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 36.Craig T, Moiseenko V, Battista J, Van Dyk J. The impact of geometric uncertainty on hypofractionated external beam radiation therapy of prostate cancer. Int J Radiat Oncol Biol Phys. 2003;57:833–842. doi: 10.1016/s0360-3016(03)00638-2. [DOI] [PubMed] [Google Scholar]