Table 1.
SAR evaluation of the amide substitution, 4a-4i.
| Compound | R | EC50 (μM) hmGluR44,a |
% Glu Max |
|---|---|---|---|
| 2 | 3,5-dichlorophenyl | 0.740 | 127 |
| 4a | 3,5-difluorophenyl | inactive | |
| 4b | 3,5-dimethylphenyl | inactive | |
| 4c | 3,5- di(trifluoromethyl)phenyl |
inactive | |
| 4d | 3,4-dimethylphenyl | inactive | |
| 4e | 3-chloro-5-fluorophenyl | 2.0 | 138 |
| 4f | 3-bromophenyl | inactive | |
| 4g | 3,4-difluorophenyl | inactive | |
| 4h | phenyl | >10 | 39 |
| 4i | benzyl | inactive | |
| 4j | 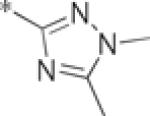 |
inactive | |
| 4k | 2-pyridyl | inactive | |
| 4l | morpholine | inactive | |
| 4m | Cyclohexyl | inactive | |
| 4n | Cyclobutyl | inactive |
Represents the average of at least one experiment performed in triplicate.
