Table 4.
| Compound | R,R′ | EC50 (μM) hmGluR44,a |
% Glu Max |
|---|---|---|---|
| 2 | -(CH2)4- | 0.75 | 127 |
| 9a | H,H | Inactive | |
| 9b | -(CH2)2- | Inactive | |
| 9c | -(CH2)3- | Inactive | |
| 9d | -(CH(CH3)2)- | Inactive | |
| 9e | 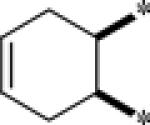 |
2.7 | 150 |
| 9f | 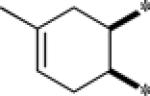 |
Inactive | |
| 9g | 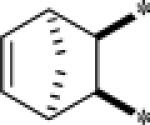 |
Inactive | |
| 9h | 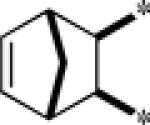 |
Inactive | |
| 9i | 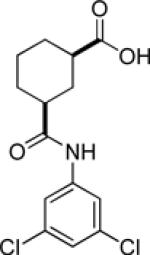 |
inactive | |
| 9j | 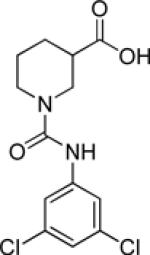 |
inactive | |
| 9k | 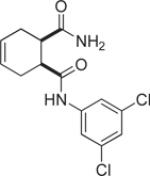 |
3.1 | 94 |
Data for inactive compounds represents the average of at least one experiment performed in triplicate; active compounds were assessed in three independent experiments in triplicate.
