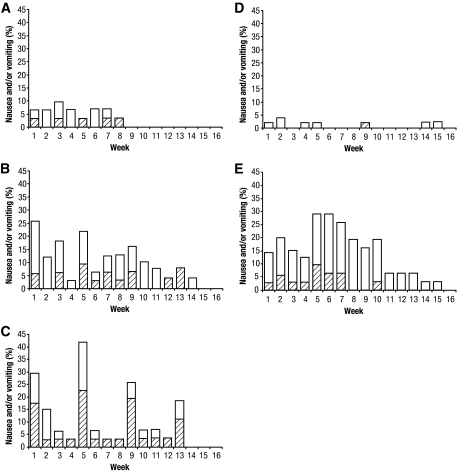
Figure 2.
Time course of nausea and vomiting as adverse events. The percentage of subjects experiencing vomiting with or without nausea (▨) or nausea (□) adverse events during each week of the 16-week trial for albiglutide 30 mg weekly (A), albiglutide 50 mg biweekly (B), albiglutide 100 mg monthly (C), placebo (D), and exenatide (E).