Abstract
OBJECTIVES:
To determine flow distribution in the cavopulmonary connections of Fontan patients with and without bilateral superior vena cavae. No large series exists that establishes the flow distributions in Fontan patients, which would be an important resource for everyday clinical use and may impact future surgical reconstruction.
METHODS:
We studied 105 Fontan patients (ages 2 - 24 years) with through-plane phase contrast velocity mapping to determine flow rates in the inferior and superior vena cava, and left and right pulmonary arteries. Superior caval anastomosis type included 40 bidirectional Glenns (of which 15 were bilateral) and 53 hemi-Fontans, while Fontan type included 69 intra-atrial baffles, 28 extracardiac conduits, and 4 atriopulmonary connections.
RESULTS:
Total caval flow was 2.9±1.0 l/min/m2, with an inferior vena cava contribution of 59%±15%. Total pulmonary flow was 2.5±0.8 l/min/m2, statistically less than caval flow and not explained by fenestration presence. The right pulmonary artery contribution (55%±13%) was, statistically greater than the left. In patients with bilateral superior cavae, the right cava accounted for 52%±14% of the flow, with no difference in pulmonary flow splits (50%±16% to the right). Age and body surface area correlated with percent inferior caval contribution (r = 0.60 and 0.74 respectively). Superior vena cava anastomosis and Fontan type did not significantly affect pulmonary flow splits.
CONCLUSIONS:
Total Fontan cardiac index was 2.9 l/min/m2, with normal pulmonary flow splits (55% to right lung). Inferior vena cava contribution to total flow increases with body surface area and age, consistent with data from healthy children.
Keywords: cardiac magnetic resonance imaging, Fontan procedure, total cavopulmonary connection, single ventricle physiology, blood flow, congenital heart disease, pediatric cardiology
In the early 1970's, Fontan and Kreutzer independently developed strategies for palliating patients with tricuspid atresia which involved baffling the caval veins directly to the pulmonary arteries.(1;2) This strategy has since been modified and adopted for the treatment of all patients with a single usable ventricle.(3-7) Staged reconstruction of the modified Fontan operation has become the standard by which single ventricle patients are palliated. Despite success at many institutions, managing these patients remains one of the most controversial and challenging aspects of pediatric cardiology.
During routine management, it may be useful to measure flow rates in various parts of the systemic venous pathway in order to make management decisions. This allows measurement of cardiac output and the determination of flow splits to the pulmonary arteries. Measurement of these flows may also be prove to be beneficial in planning future surgical reconstructions. CMR provides an accurate, non-invasive means of measuring flow rate in each limb of the cavopulmonary connection.(8;9) However, no large series exists that has used CMR to investigates the flow rates and distributions in Fontan patients. The goal of the study was to determine blood flow distribution and flow rates in these patients using CMR in the IVC, SVC, LPA and RPA. This data will serve as a guide for the clinical management of these patients. In addition, the effect of patient age and size, presence or absence of left SVC, and Fontan type on both the IVC contribution to total Fontan flow and LPA/RPA flow splits are determined.
METHODS
Patients
We studied 105 subjects with CMR phase-contrast velocity mapping. They were enrolled at either Children's Hospital of Philadelphia (CHOP) or Children's Healthcare of Atlanta (CHOA) over a six year period from March 2001 and June of 2007. All protocols were approved by the Institutional Review Boards of both institutions and informed consent obtained fom all participants.
The mean subject age was 11.5 years, ranging from 2 year to 28 years. The mean time from the Fontan operation for the 82 patients for which the data was available was 8.2 years. The SVC anastomosis and Fontan type were recorded from the medical record for each patient, when available. There were 40 patients with bidirectional Glenns, 53 with hemi-Fontans, and 12 who either had older atriopulmonary-type connections or there was insufficient information to determine the type of superior caval connection. The Fontan type included 69 intra-atrial baffles, 28 extracardiac conduits, and 4 classic atriopulmonary connections. Four patients had their surgeries at outside institutions and their Fontan types were not completely defined. There were 15 patients with bilateral SVC's. The presence of an open fenestration was recorded by the surgical note determining whether there was a surgical fenestration, and by the most recent echocardiogram documenting whether the fenestration was still patent.
All patients were required to undergo a 1-hour MRI scan. Older patients performed breath holds for image acquisition, while younger patients or patients unable to cooperate were sedated per institution protocol and averaging was used. No patient had arrhythmias that precluded imaging in the scanner. Patients were excluded if artifact precluded obtaining velocity maps in all four or five (for bilateral SVC) limbs of the Fontan pathway.
Magnetic Resonance Imaging
All CMR scans were performed at either CHOP or CHOA using either a 1.5-T Siemens Magnetom Avanto or a 1.5-T GE Signa. Velocity maps of each venous vessel (SVC, IVC, LSVC) and pulmonary artery (LPA, RPA) were obtained. In addition, aortic outflow was available in 78 patients. Five patients were excluded from the study for artifact in one or both pulmonary arteries.
Phase-encoded velocity mapping
Retrospectively gated, through-plane phase-encoded velocity maps were obtained in SVC, IVC, RPA and LPA and in most cases of the proximal ascending aorta. Care was taken to be perpendicular to flow and to obtain slice positions and orientations that were (1) distal to the azygous insertion (when still present) into the SVC, (2) proximal to the RPA upper lobe branching, and (3) distal to the aortic to pulmonary anastomosis (if present). Multiplanar reconstruction was used to set the position and angle of the imaging plane for phase encoded velocity mapping. A set of sample parameters is given in Table 1.
Table 1.
Example of velcotiy mapping parameters.
| Parameter | Setting | Parameter | Setting | |
|---|---|---|---|---|
| TR (ms) | 50 | Asymmetric echo allowed |
VENC (cm/s) | 50-150* |
| TE (ms) | 3.8 | Rectangular FOV | 66% | |
| FOV (mm) | 250 | R-R=750 ms | Acquisition window | 750 msec |
| Thickness (mm) | 5 | Flip angle (deg) | 25 | |
| Phases (calculate) | 25 | Measured phases: |
Segments | 3 |
| Matrix | 127 × 256 | Partial phase Fourier | 0 | |
| NEX | 3 | 14 | Bandwidth (HZ/px) | 260 |
50-80 used for the SVC, IVC, RPA and LPA. 150 used for the aorta.
Data Analysis
An in-house program in Matlab (The Mathworks, Natick, Masachussetts) was used to read and process acquired images. A gradient-based active contour algorithm was implemented for the semi-automatic segmentation of the vessel of interest in all the cardiac phases (10). A contour was manually outlined around the vessel of interest. This contour would automatically evolve based on gradient based forces until it identified the vessel boundary for all the cardiac phases. The segmentation was visually inspected for accuracy, and incorrect contours were adjusted manually. The segmented pixel values were converted into velocity values, which were integrated over the entire vessel area for each cardiac phase to extract flow for that phase. Mean flow rates were computed by averaging the flows through all the cardiac phases.
These flows were indexed to BSA for comparison. Fractional contribution of IVC and RPA to total blood flow were calculated and correlated with BSA and age. All population statistics are reported as a mean ± the standard deviation. Differences between statistics are reported as mean difference ± the standard error. Total pulmonary blood flow, total caval blood flow, and aortic flow were compared using a paired Student's t-test. The data were disaggregated by Fontan type, superior caval anastomosis type, and the presence or absence of an LSVC. These groups were compared by Student's t-test for independent samples to determine whether flow splits vary by group.
RESULTS
The results of the Fontan flow analysis are summarized in Table 2. The contribution of IVC flow to total systemic venous return was 59%±15%. The contribution of RPA flow to total pulmonary blood flow was 55%±13%, which was significantly greater than half (p = 0.003). Total pulmonary flow was measured at 2.4±0.7 l/min/m2, compared to 2.8 L/min/m2 for the measured total caval flow; a difference of 14% (p < 0.001).
Table 2.
Summary of velocimetry data for caval and pulmonary artery flows and comparisons between the presence and absence of an LSVC and Fontan type. All values reported as Mean±S.D.
| Flows in l/min/m2 Ratios as fraction |
No LSVC | With LSVC | LSVC vs. no LSVC |
Intracardiac Fontan |
Extracardiac Fontan |
Intra-vs. Extracardiac |
|---|---|---|---|---|---|---|
| n | 90 | 15 | 69 | 28 | ||
| Mean±S.D. | Mean±S.D. | p-value | Mean±S.D. | Mean±S.D. | p-value | |
| Age | 11.0±5.8 | 14.8±6.9 | 0.03 | 11.9±5.5 | 8.5±4.9 | 0.005 |
| BSA | 1.13±0.44 | 1.41±0.55 | 0.03 | 1.2±0.5 | 0.9±0.3 | 0.003 |
| (IVC+SVC)/BSA | 2.9±1.0 | 2.7±0.8 | 0.61 | 2.8±1.0 | 3.0±1.0 | 0.43 |
| (RPA+LPA)/BSA | 2.5±0.8 | 2.2±0.6 | 0.17 | 2.3±0.8 | 2.7±0.8 | 0.03 |
| IVC/(IVC+SVC) | 0.59±0.15 | 0.61±0.11 | 0.59 | 0.60±0.15 | 0.55±0.11 | 0.17 |
| RPA/(LPA+RPA) | 0.55±0.13 | 0.50±0.16 | 0.22 | 0.55±0.13 | 0.51±0.13 | 0.14 |
| (RPA+LPA)/(IVC+ SVC) |
0.92±0.2 | 1.1±1.7 | 0.38 | 0.88±0.3 | 1.26±1.9 | 0.1 |
| n | 67 | 11 | 52 | 22 | ||
| Aortic Flow/BSA | 3.7±1.6 | 3.2±0.7 | 0.40 | 3.4±1.5 | 4.1±1.5 | 0.11 |
| (IVC+SVC) / Ao | 0.88±0.5 | 0.88±0.2 | 0.99 | 0.92±0.5 | 0.82±0.3 | 0.41 |
| (RPA+LPA) / Ao | 0.78±0.6 | 0.73±0.3 | 0.79 | 0.80±0.6 | 0.70±0.2 | 0.48 |
In patients with bilateral SVC's, the right SVC accounted for 52%±14% of the flow. No difference was noted in the pulmonary flow splits for patients with bilateral SVC's, with 48%±19% to the right lung.
A potential source of difference between the systemic venous and pulmonary flows is the presence of a fenestration in the Fontan, which would allow a right to left shunt prior to blood reaching the branch pulmonary arteries. To investigate this, the data was disaggregated by the presence or absence of a fenestration at the time of the MRI. While the ratio of pulmonary to caval blood flow was slightly higher in patients without an open fenestration (0.86±0.24 vs. 0.84±0.20), the difference was small and not statistically significant.
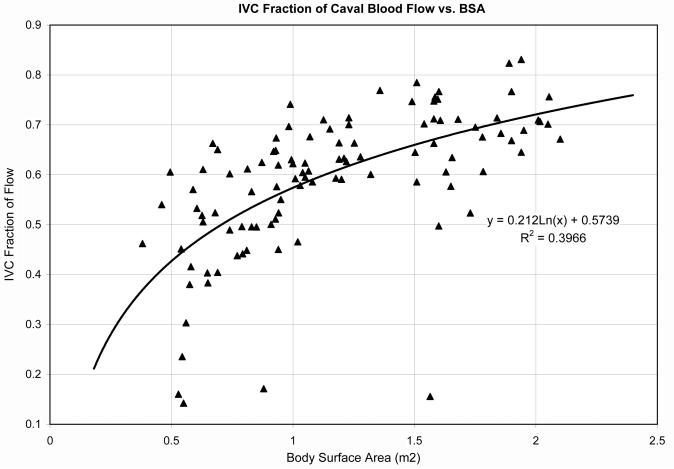
Age and BSA were correlated with the IVC fraction of total systemic venous return (r = 0.60 and 0.74 respectively, p<0.05). The IVC fraction appeared to increase in a logarithmic pattern with BSA (Figure 1). Conversely, there was no correlation between RPA fraction of pulmonary blood flow and age or BSA.
Figure 1.
Relationshiop between the IVC fraction of caval flow and body surface area. There was a strong correlation between IVC fraction and BSA; y=0.2ln(x) + 0.57, r=0.72, p<0.05.
Effect of LSVC and Fontan Type
Table 2 summarizes the comparison of patients with bilateral SVC's to those without, and with intracardiac vs. extracardiac Fontans. There was no significant effect of SVC anastomosis type, Fontan type, or presence of an LSVC on the pulmonary flow splits or the fractional contribution of the IVC to caval.
The ratio of pulmonary to caval flow is not statistically different from unity for the extracardiac Fontans. However, the mean ratio of pulmonary to caval flow for the intracardiac Fontans is statistically different from unity. The BSA and age in the extracardiac Fontans were significantly lower than that of the lateral tunnel Fontans. This reflects recent surgical trends and thus makes direct comparison of caval contributions as a function of Fontan type with regard to age and BSA difficult in this study.
Effect of ventricular morphology and type of congenital heart disease
The first part of Table 3 summarizes the flow results for patients with dominant right (62 patients) or left ventricles (37 patients). The remaining 6 patients either had a morphologically ambiguous systemic ventricle or had two good sized ventricles. There were no significant differences between the two groups. The most common types of congenital heart disease included hypoplastic left heart syndrome (42 pts), tricuspid atresia (18 pts), various forms of double outlet right ventricle (15 pts), D-TGA (8 pts), and pulmonary atresia with intact ventricular septum (7 pts). The remaing patients were a mix of more unusual substrates. The only significant finding was that the indexed caval flow for the PA-IVS group (2.2 L/min/m2) was significantly less than the HLHS group (2.8 L/min/m2). Note that the aortic flow did not follow this trend, suggesting that this group may tend to have greater aortopulmonary collateral flow.
Table 3.
Summary of velocimetry data and comparisons between the systemic LV and RV, as well as summaries of flows for breath-held vs. free breathing. All values reported as Mean±S.D.
| Flows in l/min/m2 Ratios as fraction |
RV | LV | RV vs. LV |
Breath-held | Free breathing |
Breath-hold vs. Free |
|---|---|---|---|---|---|---|
| n | 62 | 37 | 71 | 34 | ||
| Mean±S.D. | Mean±S.D. | p-value | Mean±S.D. | Mean±S.D. | p-value | |
| Age | 11.6±5.8 | 12.5±6.1 | 0.4 | 12.7±5.9 | 9.4±5.8 | 0.01 |
| BSA | 1.15±0.47 | 1.26±0.44 | 0.27 | 1.2±0.5 | 1.0±0.5 | 0.02 |
| (IVC+SVC)/BSA | 2.9±0.8 | 2.8±1.2 | 0.66 | 2.7±1.0 | 3.0±0.9 | 0.13 |
| (RPA+LPA)/BSA | 2.4±0.8 | 2.5±0.8 | 0.74 | 2.5±0.8 | 2.4±0.7 | 0.92 |
| IVC/(IVC+SVC) | 0.61±0.11 | 0.58±0.16 | 0.23 | 0.60±0.14 | 0.57±0.15 | 0.24 |
| RPA/(LPA+RPA) | 0.55±0.13 | 0.52±0.14 | 0.34 | 0.53±0.13 | 0.57±0.14 | 0.17 |
| (RPA+LPA) / (IVC+SVC) |
0.87±0.24 | 1.2±1.7 | 0.13 | 1.05±1.2 | 0.84±0.2 | 0.31 |
| n | 50 | 25 | 46 | 32 | ||
| Aortic Flow/BSA | 3.5±1.6 | 3.7±1.3 | 0.67 | 3.5±1.8 | 3.7±0.95 | 0.61 |
| (IVC+SVC) / Ao | 0.93±0.5 | 0.82±0.3 | 0.32 | 0.90±0.6 | 0.86±0.2 | 0.69 |
| (RPA+LPA) / Ao | 0.82±0.6 | 0.72±0.2 | 0.43 | 0.84±0.7 | 0.68±0.2 | 0.18 |
Effect of breath-holding on measured flows
There were 71 patients who performed breath-holding during the PC-MRI acquisitions, while 34 patients were sedated and free breathing. There were no significant differences between the two groups.
Comparing Fontan Flows to Aortic Flow
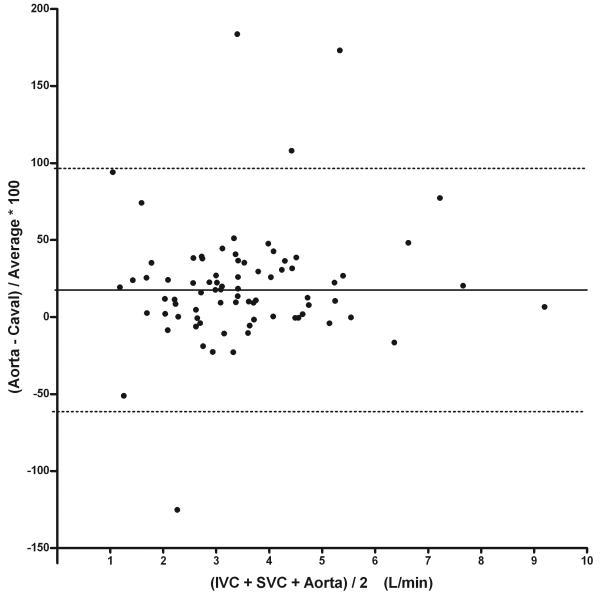
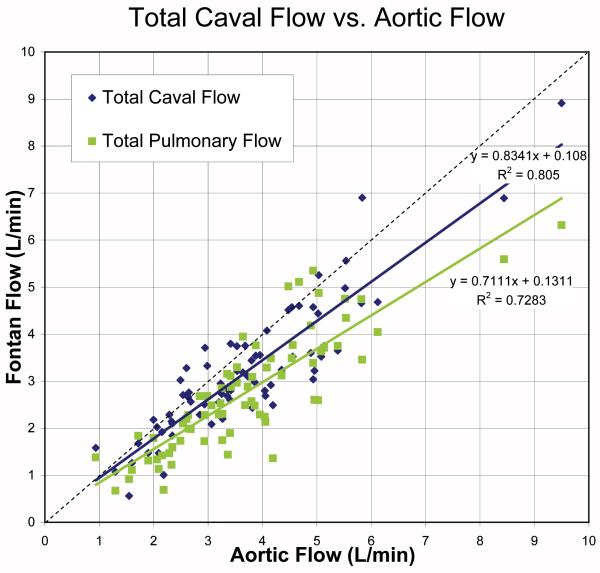
When compared to aortic flow data, the measured caval flow is generally about 12% lower across the range of measured flows (Table 4). The measured pulmonary blood flow was about 22% lower on average. This appears to be primarily a systematic rather than random difference, as Figure 2 demonstrates that there is excellent correlation between the total pulmonary blood flow, total caval blood flow, and aortic flow. Note in the Bland-Altman plot for the caval data that there are almost no data points in which the caval flow exceeds aortic flow, with most patients having measured caval flow significantly less than the aortic flow. There were 5 significant outliers in this data which were excluded from the correlation. The linear regression of caval and pulmonary flows with aortic flow demonstrates excellent correlation, but with slopes significantly less than unity.
Table 4.
Summary of velocimetry data by heart disease. All values reported as Mean±S.D.
| Flows in l/min/m2 Ratios as fraction |
HLHS | Tricuspid Atresia |
DORV | PA-IVS | D-TGA |
|---|---|---|---|---|---|
| n | 42 | 18 | 15 | 7 | 8 |
| Age | 10.0±5.0 | 11.4±6.5 | 13.6±7.1 | 15.0±3.9 | 14.9±7.4 |
| BSA | 1.09±0.42 | 1.16±0.47 | 1.24±0.49 | 1.4±0.23 | 1.3±0.6 |
| (IVC+SVC)/BSA | 2.8±0.7 | 3.2±1.4 | 3.1±1.0 | 2.2±0.8 | 3.0±1.1 |
| (RPA+LPA)/BSA | 2.3±0.7 | 2.7±1.0 | 2.9±0.9 | 2.2±0.7 | 2.4±1.1 |
| IVC/(IVC+SVC) | 0.60±0.12 | 0.54±0.17 | 0.60±0.10 | 0.62±0.21 | 0.65±0.11 |
| RPA/(LPA+RPA) | 0.56±0.12 | 0.49±0.13 | 0.51±0.08 | 0.60±0.22 | 0.50±0.11 |
| (RPA+LPA) / (IVC+SVC) |
0.85±0.26 | 0.9±0.27 | 0.95±0.21 | 1.0±0.18 | 0.82±0.21 |
| n | 32 | 10 | 12 | 4 | 4 |
| Aortic Flow/BSA | 3.3±0.8 | 3.9±1.6 | 3.7±1.5 | 3.2±0.3 | 2.8±0.6 |
| (IVC+SVC) / Ao | 0.88±0.22 | 0.88±0.16 | 0.90±0.17 | 0.78±0.10 | 1.0±0.3 |
| (RPA+LPA) / Ao | 0.74±0.21 | 0.73±0.16 | 0.83±0.20 | 0.76±0.12 | 0.7±0.1 |
Figure 2.
Top: Bland-Altman of % Difference Between Measured Aortic and Caval Flow. Note good agreement with the exception of 4 outliers, but with a bias of the aorta being 21% greater. The difference is not a function of cardiac output. Bottom: Total Caval Flow and Pulmonary Flow vs. Aortic Flow with outliers not included. Caval flow demonstrates strong correlation with aortic flow data, y=0.83x+0.11, r=0.90. Similarly, pulmonary flow demonstrates strong correlation with aortic flow (r=0.85).
DISCUSSION
The current study is the largest to date in measuring Fontan flow distribution in the systemic venous pathway. Its agreement with prior data using both mass spectrometry(11) and the Fick method(12;13) suggests that CMR can accurately measure blood flow in the Fontan baffle. The importance of a large series of patients in establishing normative data for this unique population should not be understated. Prior series are at best anecdotal and certainly cannot be viewed as establishing normal values
Many investigators, including the authors, are performing computer and in vitro modeling of the Fontan pathway in order to better understand the hemodynamics of the connection. Many investigators rely on normal values reported in the literature upon which to base their models. As the previously reported series have been quite limited, we believe the current data will provide a more comprehensive range of normal flows and flow splits upon which investigators can base their models.
This study demonstrated that total caval flow was 2.9 l/min/m2, with measured total pulmonary flow slightly less, and an inferior vena cava contribution of nearly 60%. Flows to both lungs and from both superior vena cavae, when present, were nearly equal. Type of superior vena cava anastomosis or Fontan did not significantly affect pulmonary flow splits. Age and body surface area were correlated with inferior vena cava contribution.
There was a relatively small but significant difference between measured pulmonary and systemic venous flow. Part of this may be explained by Fontan fenestration, although attempts to control for this did not account for the differences. One potential source may be in the measurement of the RPA flow, as the origin of the right upper lobe branch of the pulmonary artery is often extremely close to the SVC-RPA junction. It is therefore often very challenging to choose an imaging plane that will exclude the SVC but include the right upper lobe branch.
There was excellent correlation between aortic and both pulmonary and venous flow measurements. However, the measured systemic venous and pulmonary artery flows were significantly lower than the measured aortic flows. This may in part be explained by coronary blood flow, which is included in aortic velocity maps when measured near the valve. A more likely explanation for this difference is the presence of significant aortopulmonary collaterals, as these collaterals are known to be present in Fontan patients, especially after prolonged pleural effusions.(14) This difference deserves further investigation. The quantification of collateral flow has classically been quite difficult and this data points to a potential mechanism of quantifying this flow. Recognizing the potential differences in measured aortic and caval flow is important and has not previously been described.
Another potential source of error in the Fontan pathway flow measurements is in the breath-holding technique employed during routine PC-MRI acquisitions. It has been demonstrated that Fontan blood flow, especially the IVC, increases during inspiration and decreases during expiration(15). Many of the sequences were acquired during expiratory breath-holds, and may account for some of the differences between the aortic and Fontan baffle measurements. However, comparisons between the breath held and free breathing patients revealed no significant differences. This suggests that the effects of breath holding on the flow measurements are likely small.
The increase in IVC fraction with age shown in Figure 1 is consistent with previous studies in normal children.(16) A Doppler study by Salim et al. in 1995 looked at SVC flow compared to total pulmonary blood flow in a group of 145 healthy children. They determined that the IVC flow reached a nadir of 45% of total caval flow at 2.5 years of age and then increased to 65% of the total pulmonary blood flow by 6.6 years of age. While the IVC contributions were slightly lower than those suggested in our study, the overall trend is quite similar. Note that the initial decline in IVC fraction was not captured in our study as most of our patients were greater than 2.5 years of age, where the Salim study indicates the nadir occurs. This suggests that the presence of a Fontan does not have a significant effect on the relationship between SVC and IVC contributions of caval flow.
Prior Studies
To date, there have been very few studies which have systematically examined the flow rates and distribution in the Fontan baffle. We are not aware of any series of the magnitude currently being presented.
In 1999, Fogel et al. used CMR presaturation pulse sequences to measure the IVC and SVC flow contribution to each pulmonary artery. In this study, 10 patients underwent CMR with presaturation pulses applied individually to the SVC and IVC, with the relative flow contribution to each PA measured using the relative signal decrease. It was shown that in their cohort of 2 year olds, on average 40% of the total systemic venous return was from the IVC, with relatively more flow from the IVC directed toward the LPA. This is consistent with our study in which approximately 37% of the caval flow was from the IVC in this age group (see Figure 1). Absolute flows were not measured in their study.(17)
The largest series to date came from Rosenthal et al. in 1995. This study used respiratory mass spectrometry with acetylene to measure effective pulmonary blood flow during rest and exercise in 43 Fontan patients. The resting pulmonary blood flow was measured at 2.2 l/min/m2 for atriopulmonary Fontans and 2.3 l/min/m2 for TCPC Fontans.(11) These data agree very well with our measured total pulmonary blood flow.
Shekerdemian et al. in 1997 studied 14 Fontan patients under conditions of positive and negative pressure ventilation either immediately post-operative or during elective cardiac catheterization after the Fontan procedure. They measured pulmonary blood flow by direct Fick measurement. The pulmonary blood flow under positive pressure ventilation was 2.4±1.1 l/min/m2 and under negative pressure ventilation was 3.5±1.5 l/min/m2.(13)
Hjortdal and Pedersen et al. measured caval and pulmonary blood flow in 11 Fontan patients during rest and exercise. They measured the average total indexed caval blood flow at 2.5 l/min/m2 at rest, with 56% of the flow from the IVC at rest. The indexed pulmonary blood flow was 2.3 l/min/m2, which was not significantly different from the caval flows measured in this study.(15;18)
Limitations
CMR obtains flow data over multiple heartbeats, and the data therefore represents an average flow over the course of image acquisition (between 15 and 25 seconds). Although correlation with echocardiographic data is possible, it is difficult to draw a one-to-one comparison as echocardiography measures instantaneous flows.
The age range of our patient population was 2-24 years and therefore, large numbers in individuals in each age bracket is not present. In addition, this was a cross-sectional and not a cohort study, so how flows change in an individual patient over the course of time cannot be gleaned from this data.
This study did not concentrate on outcome based on the measured flows and is a starting point for future investigations regarding the impact of these data. Further follow-up in these patients are currently underway.
CONCLUSIONS
This study demonstrates that in Fontan patients, IVC fractional blood flow is a function of age and BSA, similar to normal children. RPA fractional flow, while there is a wide range, is on average 55% and is not a function of age or BSA. In patients with bilateral SVC's, there is no significant dominance of the right SVC overall and no significant difference in blood flow to each lung. There was no significant effect of SVC anastomosis type on pulmonary blood flow in this study. While cardiac output measured by aorta or total caval flow was not statistically different between Fontan types, measured pulmonary blood flow was significantly less in intracardiac compared to extracardiac Fontans. There were no significant differences in flows between patients with dominant morphologic left or right ventricles.
The current data demonstrates excellent correlation between all three methods of measuring cardiac output with relatively small errors between them. However, it is important to note that measuring aortic flow may actually significantly overestimate Fontan blood flow in a majority of patients with or without a fenestration. While a likely reason for this difference is aortpulmonary collaterals, this cannot be proven in the current study. This is an important finding, since measuring aortic flow may not adequately characterize the effective systemic output. This is likely better characterized by measuring caval flows, which exclude the relatively ineffective aortopulmonary collateral flow. The difference between aortic and caval flow may provide a way to quantify collateral flow in these patients, which may be an important problem contributing to hemodynamic compromise in a subset of Fontan patients. Further studies are required to determine whether this is a reliable method of quantifying aortopulmonary collateral flow.
Acknowledgments
This work was supported in part by the NIH BRP Grant R01 HL 67622 and by the Pediatric Heart Network Fontan cross-sectional study 5U01HL68279-03.
Abbreviations
- SVC
superior vena cava
- IVC
inferior vena cava
- RPA
right pulmonary artery
- LPA
left pulmonary artery
- BSA
body surface area
- Qp
pulmonary blood flow
- Qs
systemic blood flow
- CMR
cardiac magnetic resonance imaging
- PC-MRI
phase contrast magnetic resonance velocity mapping
- ROI
region of interest
- TCPC
total cavopulmonary connection
- D-TGA
transposition of the great arteries
- HLHS
hypoplastic left heart syndrome
- PA-IVS
pulmonary atresia with intact ventricular septum
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
There are no conflicts of interest to report.
Reference List
- 1.Fontan F, Baudet E. Surgical Repair of Tricuspid Atresia. Thorax. 1971;26(3):240–8. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kreutzer G, Galindez E, Bono H, de Palma C, Laura JP. An operation for the correction of tricuspid atresia. J Thorac Cardiovasc Surg. 1973;66(4):613–21. [PubMed] [Google Scholar]
- 3.Bridges ND, Lock JE, Castaneda AR. Baffle fenestration with subsequent transcatheter closure. Modification of the Fontan operation for patients at increased risk. Circulation. 1990;82(5):1681–9. doi: 10.1161/01.cir.82.5.1681. [DOI] [PubMed] [Google Scholar]
- 4.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96(5):682–95. [PubMed] [Google Scholar]
- 5.di Carlo D, Williams WG, Freedom RM, Trusler GA, Rowe RD. The role of cava-pulmonary (Glenn) anastomosis in the palliative treatment of congenital heart disease. J Thorac Cardiovasc Surg. 1982;83(3):437–42. [PubMed] [Google Scholar]
- 6.Kawashima Y, Kitamura S, Matsuda H, Shimazaki Y, Nakano S, Hirose H. Total cavopulmonary shunt operation in complex cardiac anomalies. A new operation. J Thorac Cardiovasc Surg. 1984;87(1):74–81. [PubMed] [Google Scholar]
- 7.Norwood WI, Lang P, Casteneda AR, Campbell DN. Experience with operations for hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 1981;82(4):511–9. [PubMed] [Google Scholar]
- 8.Beerbaum P, Korperich H, Barth P, Esdorn H, Gieseke J, Meyer H. Noninvasive Quantification of Left-to-Right Shunt in Pediatric Patients : Phase-Contrast Cine Magnetic Resonance Imaging Compared With Invasive Oximetry. Circulation. 2001;103(20):2476–82. doi: 10.1161/01.cir.103.20.2476. [DOI] [PubMed] [Google Scholar]
- 9.Beerbaum P, Korperich H, Gieseke J, Barth P, Peuster M, Meyer H. Rapid Left-to-Right Shunt Quantification in Children by Phase-Contrast Magnetic Resonance Imaging Combined With Sensitivity Encoding (SENSE) Circulation. 2003;108(11):1355–61. doi: 10.1161/01.CIR.0000087603.97036.C2. [DOI] [PubMed] [Google Scholar]
- 10.Kozerke S, Botnar R, Oyre S, Scheidegger MB, Pedersen EM, Boesiger P. Automatic vessel segmentation using active contours in cine phase contrast flow measurements. J Magn Reson Imaging. 1999;10(1):41–51. doi: 10.1002/(sici)1522-2586(199907)10:1<41::aid-jmri6>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal M, Bush A, Deanfield J, Redington A. Comparison of Cardiopulmonary Adaptation During Exercise in Children After the Atriopulmonary and Total Cavopulmonary Connection Fontan Procedures. Circulation. 1995;91(2):372–8. doi: 10.1161/01.cir.91.2.372. [DOI] [PubMed] [Google Scholar]
- 12.Shachar GB, Fuhrman BP, Wang Y, Lucas RV, Jr., Lock JE. Rest and exercise hemodynamics after the Fontan procedure. Circulation. 1982;65(6):1043–8. doi: 10.1161/01.cir.65.6.1043. [DOI] [PubMed] [Google Scholar]
- 13.Shekerdemian LS, Bush A, Shore DF, Lincoln C, Redington AN. Cardiopulmonary Interactions After Fontan Operations : Augmentation of Cardiac Output Using Negative Pressure Ventilation. Circulation. 1997;96(11):3934–42. doi: 10.1161/01.cir.96.11.3934. [DOI] [PubMed] [Google Scholar]
- 14.Triedman JK, Bridges ND, Mayer JE, Jr., Lock JE. Prevalence and risk factors for aortopulmonary collateral vessels after Fontan and bidirectional Glenn procedures. J Am Coll Cardiol. 1993;22(1):207–15. doi: 10.1016/0735-1097(93)90836-p. [DOI] [PubMed] [Google Scholar]
- 15.Hjortdal VE, Emmertsen K, Stenbog E, Frund T, Schmidt MR, Kromann O, et al. Effects of exercise and respiration on blood flow in total cavopulmonary connection: a real-time magnetic resonance flow study. Circulation. 2003;108(10):1227–31. doi: 10.1161/01.CIR.0000087406.27922.6B. [DOI] [PubMed] [Google Scholar]
- 16.Salim MA, DiSessa TG, Arheart KL, Alpert BS. Contribution of Superior Vena Caval Flow to Total Cardiac Output in Children : A Doppler Echocardiographic Study. Circulation. 1995;92(7):1860–5. doi: 10.1161/01.cir.92.7.1860. [DOI] [PubMed] [Google Scholar]
- 17.Fogel MA, Weinberg PM, Rychik J, Hubbard A, Jacobs M, Spray TL, et al. Caval Contribution to Flow in the Branch Pulmonary Arteries of Fontan Patients With a Novel Application of Magnetic Resonance Presaturation Pulse. Circulation. 1999;99(9):1215–21. doi: 10.1161/01.cir.99.9.1215. [DOI] [PubMed] [Google Scholar]
- 18.Pedersen EM, Stenbog EV, Frund T, Houlind K, Kromann O, Sorensen KE, et al. Flow during exercise in the total cavopulmonary connection measured by magnetic resonance velocity mapping. Heart. 2002;87(6):554–8. doi: 10.1136/heart.87.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]