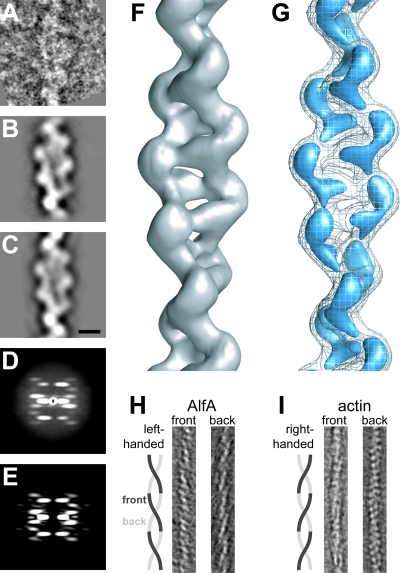
FIG. 6.
Three-dimensional reconstruction of single AlfA filaments. (A) Representative segment of an AlfA filament in a negatively stained micrograph. The conditions used were as follows: 1.25 M KCl, 25 mM Tris HCl (pH 7.5), 1 mM MgCl, 1 mM DTT, 25°C, 5 mM AMP-PNP, and 7 μm AlfA. (B) Average of 757 AlfA segments after iterative helical real-space reconstruction. (C) Reprojection of the final AlfA filament model in the same orientation as the average shown in panel B. Scale bar, 5 nm. (D) Average power spectrum for 2,000 nonoverlapping segments. (E) The two-dimensional power spectrum of the final AlfA filament model is very similar to the average power spectrum shown in panel D. (F) Three-dimensional reconstruction of an AlfA filament, contoured to enclose the expected mass of the AlfA subunits. (G) AlfA filament at the same contour as the contour shown in panel F (mesh) and at a higher contour level (blue), clearly showing the separation between the two strands of the filament and between AlfA subunits in each strand. (H) Approximately 3.5-nm slices from a tomographic reconstruction of a single AlfA filament, demonstrating that the long pitch of the helix is left-handed. (I) Tomogram of actin, prepared like the tomogram for AlfA in panel H as a control, demonstrating the well-established right-handed pitch of the long helix. In panels H and I the width of the image is 25 nm.