Abstract
The administration of vectors designed to elicited cell-mediated immune responses may have other consequences that are clinically significant. To explore this possibility, we evaluated T-cell activation during the first 2 months after recombinant adenovirus serotype 5 (rAd5) prime or boost immunizations in rhesus monkeys. We also evaluated the kinetics of T-lymphocyte activation in both the systemic and the mucosal compartments after rAd5 administration in monkeys with preexisting immunity to Ad5. The rAd5 immunization induced lower-frequency Gag epitope-specific CD8+ T cells in the colonic mucosa than in the peripheral blood. There was evidence of an expansion of the simian immunodeficiency virus Gag-specific CD8+ T-cell responses, but not the Ad5 hexon-specific T-cell responses, following a homologous rAd5 boost. A striking but transient T-lymphocyte activation in both the systemic and the mucosal compartments of rhesus monkeys was observed after rAd5 immunization. These findings indicate that the administration of a vaccine vector such as Ad5 can induce a global activation of T cells.
Considerable effort has been invested in the development of vaccine strategies for eliciting cell-mediated immune responses to human immunodeficiency virus (HIV). Studies in simian immunodeficiency virus (SIV)/SHIV-infected nonhuman primates and HIV-infected humans demonstrated a central role for cell-mediated immune responses in the containment of HIV replication (1, 12). These findings led to the hypothesis that vaccine-elicited cell-mediated immunity might contribute to improved control of HIV in infected individuals. Studies in the SIV and SHIV/macaque models have supported this hypothesis, demonstrating a decrease in peak plasma virus RNA levels during primary infection, protection against memory CD4+ T-cell lymphocyte loss, and prolonged survival of monkeys that had vaccine-elicited cell-mediated immunity to the virus prior to challenge (8, 15, 16).
Despite promising results in preclinical nonhuman primate studies, a prophylactic HIV vaccine trial of the Merck recombinant adenovirus serotype 5 (rAd5) vector expressing HIV gag, pol, and nef genes (STEP trial) was recently halted due to a 2.3-fold increase of HIV acquisition in vaccinees with preexisting neutralizing antibodies (NAbs) to Ad5 (2, 9, 10). This finding raised the possibility that T lymphocytes that are activated in response to vaccination might represent an increased pool of potential targets for HIV infection, and the persistence of such activated cells may increase the susceptibility of the vaccinated individual to acquiring an HIV infection (5, 11). HIV replicates most readily in activated, CCR5+CD4+ T lymphocytes. It has been suggested that vaccines that elicit potent cellular immune responses may also activate subpopulations of CD4+ T lymphocytes. In fact, in the aftermath of the failed STEP trial, it was proposed that the activation of Ad5-specific T cells in individuals with prior Ad5 immunity may have contributed to their increased acquisition of HIV after vaccination.
The contribution of cellular activation in mucosal tissues to acquisition of HIV remains unexplored (2). HIV transmission occurs most often across mucosal barriers. There is increasing evidence that CD4+ T lymphocytes are among the first cells infected during the transmission event (4). Activation of mucosal populations of lymphocytes as a consequence of vaccination could contribute to increasing the incidence of HIV transmission at a mucosal site.
To examine these issues, the present study was initiated to explore vaccine-induced activation of T-lymphocyte populations in rhesus monkeys. The character and kinetics of the activation of both circulating and mucosal T-lymphocyte populations were evaluated after immunization with a variety of immunogens. These experiments demonstrate a striking but transient T-lymphocyte activation induced by adenovirus-based vaccine vectors in both the systemic and mucosal compartments of rhesus monkeys.
MATERIALS AND METHODS
Peripheral blood and colon biopsy specimens.
Peripheral blood and colon biopsy specimens were obtained from rhesus monkeys (Macaca mulatta). All animals were maintained in accordance with the American Association for Accreditation of Laboratory Animal Care guidelines.
Immunization of rhesus monkeys.
rAd5 vaccine constructs were constructed as previously described and administered by intramuscular injection using a needle and syringe (7).
Isolation of lymphocytes from colon biopsy specimens.
Colon biopsy specimens were incubated with Hanks balanced salt solution supplemented with 1 mM EDTA and 5% fetal bovine serum at 37°C for 30 min with shaking. Mucosal tissues were then digested by incubation at 37°C in RPMI 1640 containing 5% fetal bovine serum supplemented with type II collagenase (Sigma-Aldrich) at 100 U/ml with shaking. The biopsy tissues were then disrupted by aspirating tissue pieces in and out of a syringe, and released cells were filtered through a nylon mesh screen. Lymphocytes were purified with a Percoll gradient at 1,000 × g for 20 min. Lymphocytes were collected between the 30 and 65% Percoll layers and washed two times before processing for staining.
Tetramer, T-cell maturation, and activation-associated molecules.
The antibodies used in the present study were directly coupled to fluorescein isothiocyanate, phycoerythrin (PE), PE-Texas Red, peridinium chlorophyll protein-Cy5.5 (PerCP-Cy5.5), PE-Cy7, AmCyan, Pacific Blue, allophycocyanin, Alexa Fluor 700, and Quantum-Dot 605. All reagents were validated and titrated using rhesus monkey peripheral blood lymphocytes (PBL). The tetrameric Mamu-A*01/p11C, C-M complex (CTPYDINQM) (6) was used in conjugation with CD3 (SP34.2), CD4 (L200), CD8 (SK1), CD28 (L293), CD95 (DX2), CCR5 (3A9), and PD-1 specific antibodies to identify activated CD4+, CD8+, and p11C+ CD8+ T cells from peripheral blood and colonic mucosa. Cells were then fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), stained with antibodies specific for Ki67 (B56), and fixed with 1% formaldehyde. A violet fluorescent reactive dye (ViViD; Invitrogen) was also used as a viability marker to exclude dead cells in the analysis. Unconjugated anti-CD4 antibody was obtained from BD Bioscience, and Quantum-Dot 605 was obtained from Invitrogen. All other monoclonal antibodies (MAbs) were purchased from BD Biosciences unless otherwise indicated.
PBL stimulation and intracellular cytokine staining.
PBL were isolated from EDTA-anticoagulated blood and frozen in the vapor phase of liquid nitrogen. Cells were later thawed and allowed to rest for 6 h at 37°C in a 5% CO2 environment. Two million PBL were incubated for 6 h with medium as the negative control, overlapping 15-mer peptides spanning SIV Gag, SIV Env, HIV Env, and the Ad5 hexon protein, or staphylococcal enterotoxin B (SEB; Sigma-Aldrich) as the positive control. All cultures contained Monensin (GolgiStop; BD Biosciences), as well as 1 μg of anti-CD28/ml and 1 μg of anti-CD49d/ml (BD Biosciences). The cultured cells were then stained with pretitered quantities of CD3, CD4, CD8, and CCR5 specific MAbs. After being fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences), cells were stained with antibodies specific for Ki67, gamma interferon (IFN-γ; B27), tumor necrosis factor alpha (TNF-α; MAb11), and interleukin-2 (IL-2; MQ1-17H12), and fixed with 1% formaldehyde. Samples were collected on a LSR II instrument (BD Biosciences) and analyzed by using FlowJo software (Tree Star). Approximately 106 events were collected per sample. Doublets were excluded by forward-scatter (FSC) area versus FSC height. Dead cells were excluded by their staining with a violet fluorescent reactive dye. CD4+ and CD8+ T cells were defined by their expression of CD3, CD4, and CD8. The background level of cytokine staining varied from sample to sample, but was typically below 0.02% of gated CD4+ or CD8+ T lymphocytes. All values used for analysis were background subtracted.
Ad-specific NAb assays.
NAbs to Ad5 were determined by luciferase-based virus neutralization assays as previously described (14). Neutralization titers were defined as the maximum serum dilution that neutralized 90% of the luciferase activity.
RESULTS
Transient T-cell activation following rAd5 prime.
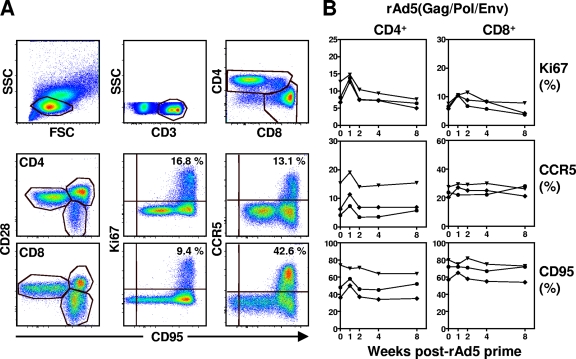
We first assessed peripheral blood T-cell activation in three rhesus monkeys that received 1010 particles each of two replication-defective rAd5 constructs, one expressing SIVmac239 Gag/Pol and the other expressing SIVmac239 Env. Peripheral blood mononuclear cell expression of Ki67, CCR5, and CD95 was evaluated on a regular basis for 8 weeks. As shown in Fig. 1, rAd5 vaccination resulted in a twofold increase of Ki67 expression and a marginal increase of CCR5 and CD95 expression on total CD4+ T cells at week 1. However, the transient expression of these activation-associated molecules was returning to baseline by week 2. In addition, rAd5 immunization also resulted in a modest increase of Ki67 expression with no significant changes of CCR5 and CD95 expression on the total CD8+ T cells 1 week after immunization. These data suggest that there is a short-term T-cell activation during the first 2 weeks after a primary rAd5 immunization.
FIG. 1.
CD4+ and CD8+ T-cell activation following rAd5 priming. (A) CD4+ and CD8+ T cells were identified by first gating on lymphocytes and then gating on CD3+amine− cells that were either CD4+ or CD8+. The percentages of Ki67+, CCR5+, CD28+, or CD95+ (total memory) CD4+ and CD8+ T cells are shown in a representative rhesus monkey. (B) Ki67, CCR5, and CD95 expression on CD4+ and CD8+ T cells in the peripheral blood of three rhesus monkeys after rAd5 priming.
Transient T-cell activation following rAd5 boost.
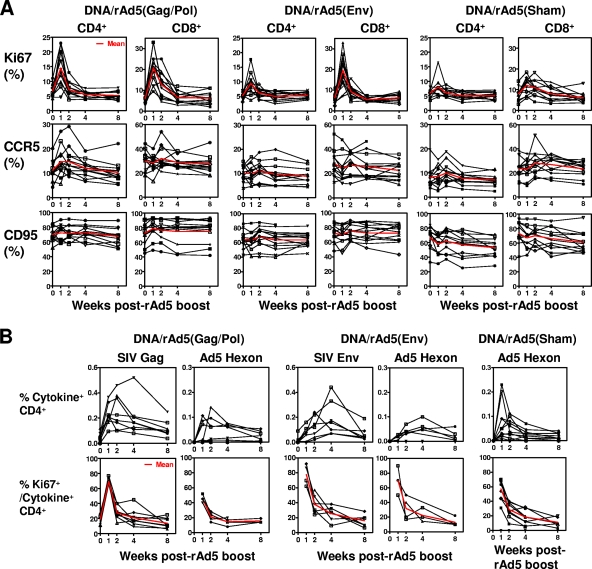
We next sought to determine whether T-cell activation also occurred after a rAd5 boost immunization. Three groups of rhesus monkeys that were previously primed with plasmid DNA immunogens were evaluated for a period of 8 weeks after a rAd5 boost. In the DNA/rAd5(Gag/Pol) group, 13 monkeys received 1011 particles of a rAd5 construct expressing SIVmac239 Gag/Pol as a boosting immunization. In the DNA/rAd5(Env) group, 13 monkeys received 1011 particles of a rAd5 construct expressing SIVmac239 Env as a boosting immunization. Another 13 control animals received 1011 particles of rAd5 with no insert (sham). Total CD4+ or CD8+ T cells that express Ki67, CCR5, or CD95 were evaluated on a regular basis for 8 weeks after rAd5 boost. As shown in Fig. 2A, the CD4+ T cells of monkeys that received the rAd5(Gag/Pol) boost showed a threefold increase of Ki67 expression, a marginal increase of CCR5 expression, and no significant change of CD95 expression. A fourfold increase of Ki67 expression with no significant change in CCR5 and CD95 expression was detected on the total CD8+ T-cell population of these monkeys. However, this dramatic T-cell immune activation, as defined by Ki67 expression, returned to baseline by 2 to 3 weeks after the rAd5 boost. Interestingly, only marginal increases of Ki67 expression were detected on both CD4+ and CD8+ T cells in the group of animals that received the sham rAd5 boost. These results suggest that the transient T-cell activation induced by the rAd5 immunization may not be caused by the rAd5 vector itself but rather by the vector in association with the expressed transgene product.
FIG. 2.
CD4+ and CD8+ T-cell activation following rAd5 boost. (A) Three groups of rhesus monkeys that were previously primed with plasmid DNA were evaluated after rAd5 boost: (i) DNA/rAd5(Gag/Pol), monkeys boosted with rAd5 expressing SIV Gag and Pol; (ii) DNA/rAd5(Env), monkeys boosted with rAd5 expressing SIV Env; and (iii) control, monkeys boosted with empty rAd5 vector (sham). Ki67, CCR5, and CD95 expression on total CD4+ and CD8+ T cells were evaluated for each individual animal on a regular basis after the rAd5 boost. The mean value for all 13 animals in each experimentally vaccinated group is shown in red. (B) Activation of SIV- and Ad5-specific CD4+ T cells following rAd5 boost. PBL isolated from three different cohorts of rhesus monkeys at the indicated times after rAd5 boost were exposed to pools of overlapping peptides spanning the SIV Gag, Env, or Ad5 hexon proteins and the fractions of CD4+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining. The sum total production of all three cytokines for CD4+ T cells of each individual animal is shown in the top panels. The percentage of Gag-, Env-, or hexon-specific cytokine-producing CD4+ T cells that express Ki67 is shown in the bottom panels, and the mean value is indicated in red.
Activation of SIV- and Ad5-specific CD4+ T cells following rAd5 boost.
Having demonstrated a transient activation of total CD4+ and CD8+ T cells after rAd5 immunization, we evaluated the activation of the vaccine-elicited antigen-specific CD4+ T cells. PBL isolated from the monkeys after rAd5 boost immunization were exposed to pools of overlapping peptides spanning the SIV Gag, SIV Env, and Ad5 hexon proteins, and the percentages of CD4+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining (Fig. 2B, top panels). In monkeys receiving the rAd5(Gag/Pol) immunogen, Gag-specific CD4+ T cells expanded until 2 weeks after boost. Hexon-specific CD4+ T cells were maximal at week 1 after immunization. These Gag- and hexon-specific CD4+ T cells had a three- to fourfold increase of Ki67 expression 1 week after rAd5 boost (Fig. 2B, bottom panels). Monkeys that received a rAd5(Env) boost also had an expansion of the Env-specific and hexon-specific CD4+ T cells, and an increase of Ki67 expression was detected on these antigen-specific CD4+ T cells during the first 2 weeks after the boost. Activation of the hexon-specific CD4+ T cells in the sham control vaccinated animals showed similar kinetics. These results suggest that vaccine-elicited antigen-specific CD4+ T cells, like the total CD4+ T-cell population, were transiently activated after the rAd5 boost.
T cell activation following plasmid DNA priming immunization.
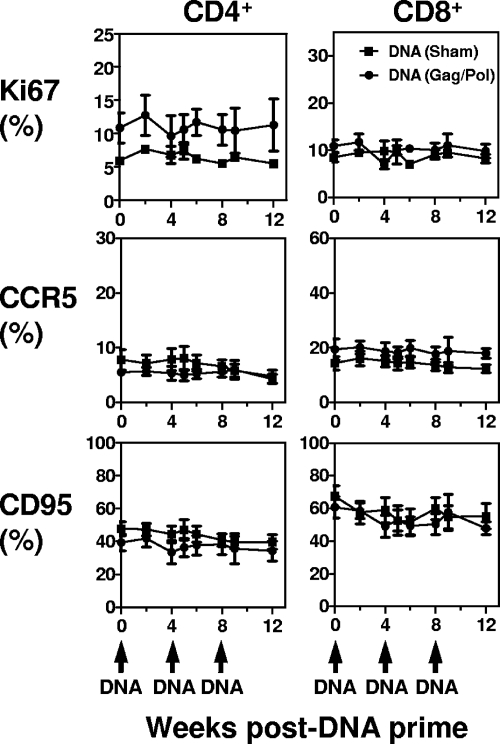
We then sought to determine whether this transient T-cell activation could be detected in animals receiving other immunogens. Two groups of rhesus monkeys were primed with plasmid DNA and monitored for 8 weeks to evaluate their CD4+ and CD8+ T-cell activation status. Six animals were primed with a plasmid DNA vaccine construct expressing SIV Gag and Pol at weeks 0, 4, and 8. Another six control animals received a sham plasmid DNA prime according to the same schedule. Ki67, CCR5, and CD95 expression on CD4+ and CD8+ T cells was evaluated for each animal for 12 weeks (Fig. 3). None of these immune activation associated molecules showed increased expression on the CD4+ or CD8+ T cells in either group of animals after plasmid DNA immunization.
FIG. 3.
CD4+ and CD8+ T-cell activation following plasmid DNA priming. Two groups of rhesus monkeys were evaluated following plasmid DNA prime: (i) sham, six monkeys primed with sham plasmid DNA and (ii) Gag/Pol, six monkeys primed with plasmid DNA expressing SIV Gag and Pol. Ki67, CCR5, and CD95 expression on CD4+ and CD8+ T cells was evaluated for each individual animal on a regular basis after plasmid DNA prime. The data are presented as the mean frequencies of Ki67+, CCR5+, and CD95+ CD4+ or CD8+ T cells from each group of monkeys ± the standard error of the mean (SEM).
T-cell immune activation following rAd35 priming.
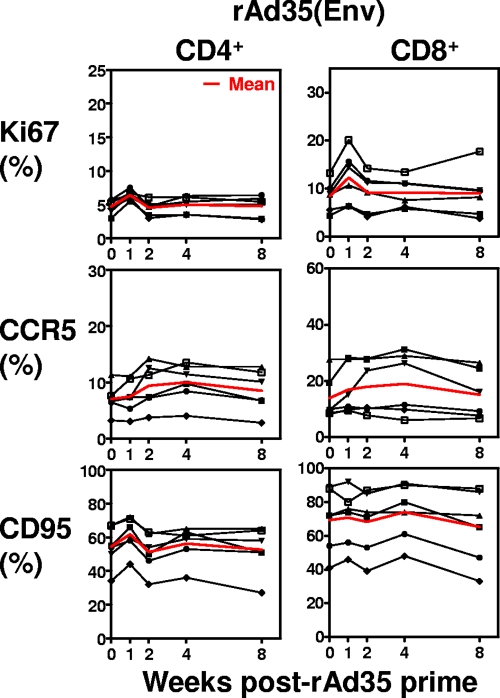
We also evaluated T-cell activation after immunization with the rare serotype Ad vector Ad35 (Fig. 4). Six rhesus monkeys were primed with 1011 particles of rAd35 expressing HIV Env and were monitored for 8 weeks to evaluate their CD4+ and CD8+ T-cell activation. Ki67 expression increased modestly on CD4+ and CD8+ T cells 1 week after immunization. No consistent changes in CCR5 and CD95 expression by these cell populations were observed. This modest T-cell activation induced by rAd35 immunization may be a consequence of the modest potency of the vaccine vector as a stand-alone immunogen.
FIG. 4.
CD4+ and CD8+ T-cell activation following rAd35 priming. Ki67, CCR5, and CD95 expression on CD4+ and CD8+ T cells was evaluated for six animals on a regular basis after rAd35 prime. The mean value for all six animals is shown in red.
Systemic and mucosal T-cell activation following repeated rAd5 immunizations.
It has been suggested that Ad5-specific CD4+ T cells in Ad5 seropositive subjects could be activated after rAd5 immunization, thereby providing increased numbers of potential target cells for HIV-1 infection. To explore this possibility, we evaluated the immune activation status of total T cells and antigen-specific T cells after repeated rAd5 immunizations (Fig. 5). Because of its proven safety and immunogenicity, Mycobacterium bovis bacillus Calmette-Guérin (BCG) is being explored as a vaccine vector to confer protection against infectious pathogens and cancer. In the present study, seven Mamu-A*01+ rhesus monkeys received rBCG inoculations at weeks 0 and 23, followed by boosting immunizations with 1010 particles of both rAd5 expressing SIV Gag/Pol and rAd5 expressing Env at weeks 43 and 133 (3). Ki67, CCR5, PD-1, and CD95 expression on CD4+ and CD8+ T cells were evaluated on a regular basis for 8 weeks after the two rAd5 boosts. As shown in Fig. 5B, the first rAd5 boost induced a transient increase of Ki67 expression on both CD4+ and CD8+ T cells. This first rAd5 immunization also elicited high titer Ad5-specific NAbs with a mean 90% neutralization titer of 2,136 in the seven monkeys (Table 1).
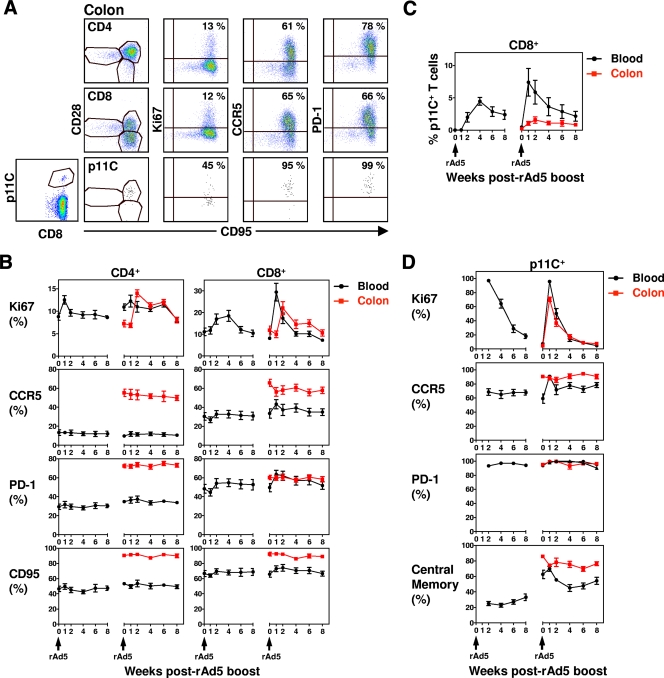
FIG. 5.
T-cell activation following two rAd5 boosts. (A) Expression of Ki67, CCR5, PD-1, CD28, and CD95 on mucosal CD4+, CD8+, and p11C+ CD8+ T cells from a representative rhesus monkey. (B) Seven animals were previously primed with rBCG expressing SIV Gag, Pol, and Env. Ki67, CCR5, PD-1, and CD95 expression on CD4+ and CD8+ T cells was evaluated for each individual animal from both PBL and colon biopsies on a regular basis after two rAd5 boosts (1.5 years apart). The data are presented as the mean frequencies of Ki67+, CCR5+, PD-1+, and CD95+ CD4+ or CD8+ T cells in PBL and colon biopsies for all seven monkeys ± the SEM. (C) Mean frequency of p11C+CD8+ T cells in PBL and colon biopsies for all seven monkeys ± the SEM. (D) Percentage of p11C+CD8+ T cells that express Ki67, CCR5, PD-1, and CD28+ CD95+ (central memory) was evaluated in PBL and colon biopsies, and the means ± the SEM are shown.
TABLE 1.
Ad5 serotiter before rAd5 boost
| Animal | Ad5 90% neutralization titer |
|---|---|
| 152-06 | 1,094 |
| 157-06 | 1,750 |
| 207-06 | 4,214 |
| 208-06 | 2,446 |
| 210-06 | 1,288 |
| 211-06 | 1,837 |
| 214-06 | 2,329 |
These animals then received a second rAd5 boost, and T-cell activation status was evaluated in both PBL and colonic mucosa. Compared to the T cells in the peripheral blood, the CD4+ and CD8+ T cells in the colonic mucosa had a different maturation, activation, and apoptosis status. In contrast to these cells in the peripheral blood, the majority of the CD4+ T cells isolated from the colonic mucosa were CD95+ memory CD4+ T cells expressing CCR5 and the programmed death-associated PD-1 molecule. Most of the CD8+ T cells in the colonic mucosa were also memory CD8+ T cells expressing CCR5 and PD-1 (Fig. 5A and B). In addition, mucosal CD4+ and CD8+ T cells displayed a distinct kinetics of activation after the second rAd5 boost. In the peripheral blood, the second rAd5 boost induced a modest increase of Ki67 expression on CD4+ T cells and a threefold increase of Ki67 expression on CD8+ T cells 1 week after immunization (Fig. 5B). In the colonic mucosa, Ki67 expression was maximal on T cells at week 2, 1 week later than its peak in the peripheral blood, and the expression remained at an elevated level for 3 to 4 weeks after vaccination. Therefore, in animals with preexisting NAbs to Ad5, Ad5 immunization induced a transient T-cell activation in the peripheral blood and a delayed, more sustained T-cell activation in the colonic mucosa. These results highlight the potential importance of evaluating mucosal T-cell activation in HIV-1 vaccine studies.
We also evaluated the activation status of the antigen-specific CD8+ T cells, as defined by p11C Mamu-A*01 tetramer staining in the peripheral blood and colonic mucosa cells of these monkeys. As shown in Fig. 5C, p11C+CD8+ T cells were initially detected in the peripheral blood at week 2, and they continued to expand in number until 4 weeks after the first Ad5 boost. After the second Ad5 boost, the p11C+ CD8+ T-cells expanded quickly to a median value of 7% at week 1. However, considerably fewer p11C+CD8+ T cells were detected in the colonic mucosa after these immunizations, with a peak response of 1.5%. Therefore, the rAd5 immunizations induced lower frequency p11C+CD8+ T cells in the colonic mucosa than in the peripheral blood. We then compared the activation status of p11C+ CD8+ T cells in the peripheral blood and colonic mucosa after the rAd5 boosting immunizations. Interestingly, the p11C+ CD8+ T cells in the colonic mucosa were predominantly CD28+ CD95+ memory CD8+ T cells that expressed high levels of CCR5 and PD-1 (Fig. 5A and D). In addition, the second rAd5 boost induced comparable Ki67 expression on p11C+CD8+ T cells in the peripheral blood and colonic mucosa.
Finally, we evaluated the activation status of the SIV Gag-specific CD4+ and CD8+ T cells after the two rAd5 boosts. We have previously reported that antigen-specific CD4+ or CD8+ T cells were almost undetectable in the peripheral blood after a rBCG priming immunization (3). After the first rAd5 boost, as shown in Fig. 6, SIV Gag-specific CD4+ T cells expanded, and this response was maximal at week 1. Antigen-specific CD8+ T cells were initially detected at week 2 and continued to increase until 8 weeks after the boost. After the second rAd5 boost, in the presence of preexisting immunity to Ad5, SIV Gag-specific CD4+ T cells showed only a modest increase at week 1. However, a dramatic expansion of the Gag-specific CD8+ T cells was detected at week 1, and this response persisted at a high level until 8 weeks after the boost. These results indicate that Gag-specific CD8+ T-cell responses were not blunted by preexisting Ad5-specific NAbs in the present study.
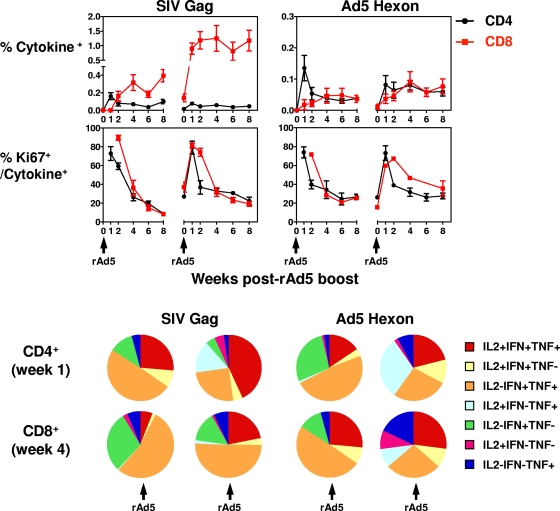
FIG. 6.
SIV- and Ad5-specific CD4+ and CD8+ T cells after two rAd5 boosts. Seven animals previously primed with rBCG expressing SIV Gag, Pol, and Env were immunized with rAd5 expressing SIV Gag, Pol and Env. PBL isolated from these rhesus monkeys at the indicated time points were exposed to pools of overlapping peptides spanning the SIV Gag or Ad5 hexon protein, and the fractions of CD4+ and CD8+ T cells producing IFN-γ, TNF-α, or IL-2 were determined by intracellular cytokine staining. The sum total production of all three cytokines by the noted lymphocyte subpopulation is presented as mean ± the SEM of all seven animals in the top panels. The percentages of Gag- and hexon-specific cytokine-producing CD4+ or CD8+ T cells that express Ki67 are shown in the middle panels. The total antigen-specific CD4+ (evaluated at 1 week) or CD8+ T cells (evaluated at 4 weeks) after rAd5 boosts were divided into seven distinct populations based on their production of IFN-γ, TNF-α, and IL-2. The cytokine profiles of these cells were determined by expressing each cytokine response as a proportion of the total antigen-specific cytokine-producing CD4+ or CD8+ T-cell response. The data are presented as the mean values of seven animals in a pie chart in the bottom panels.
We then evaluated the cellular immune response to the Ad5 vector. Hexon-specific CD4+ T cells peaked at 1 week after the first Ad5 immunization. Hexon-specific CD8+ T cells expanded slowly and were maintained at a low level following the first rAd5 immunization. However, there was no further expansion of the hexon-specific CD4+ and CD8+ T cells after the second rAd5 immunization. These results suggest that preexisting immunity to Ad5 blunted the hexon-specific CD4+ and CD8+ T-cell responses after rAd5 vaccination. We also evaluated the activation status of the antigen-specific T cells. As shown in the middle panels of Fig. 6, a substantial but transient increase of Ki67 expression on both Gag- and hexon-specific CD4+ and CD8+ T cells was detected during the first 2 weeks after each rAd5 boost.
Finally, we evaluated the functional profiles of the rAd5-induced antigen-specific T cells. rAd5-induced total antigen-specific CD4+ (evaluated at 1 week) or CD8+ T cells (evaluated at 4 weeks) were divided into seven distinct populations based on their production of IFN-γ, TNF-α, and IL-2. The cytokine profiles of these cells were determined by expressing each cytokine response as a proportion of the total antigen-specific cytokine-producing CD4+ or CD8+ T-cell response. The data are presented as the mean values of seven animals in a pie chart in the bottom panels of Fig. 6. A total of 25% of the Gag-specific CD4+ T cells evaluated at 1 week after the first rAd5 boost were IFN-γ+ TNF-α+ IL-2+, and these triple-cytokine-positive cells expanded to 50% of the Gag-specific CD4+ T cells response 1 week after the second boost. In addition, triple cytokine positive Gag-specific CD8+ T cells evaluated at 4 weeks after immunization also expanded from 5% after the first rAd5 boost to 25% after the second rAd5 boost. However, there was no significant expansion of the triple cytokine positive hexon-specific CD4+ or CD8+ T cells after the second rAd5 boost. These results indicate that in the presence of preexisting immunity to Ad5, the polyfunctionality of the Gag-specific CD4+ and CD8+ T cells continued to expand after repeated rAd5 vaccinations.
DISCUSSION
In the aftermath of the failed STEP trial, it was suggested that T cells activated in response to a rAd5 vaccine might represent an increased pool of potential targets for HIV infection, and the persistence of such activated cells may increase the susceptibility of vaccinated individuals to HIV infection. To explore this hypothesis, several laboratories evaluated the activation status of T cells in subjects who had received rAd5-based vaccines in the presence or absence of preexisting NAbs to rAd5 (5, 10, 11). Many of these studies found no evidence of T-cell activation after rAd5 immunization in Ad5-seronegative or -seropositive subjects. However, in most of these studies, human peripheral blood mononuclear cells were not available for analysis during the first weeks after rAd5 immunization.
In the present study we evaluated T-cell activation status on a regular basis during the first 2 months after rAd5 prime or rAd5 boost immunization in a rhesus monkey model. Our results demonstrate that T-cell activation occurs during the first 14 days after rAd5 immunization. These findings are consistent with those of McElrath (9), who demonstrated a similar transient activation of T cells in the days after a rAd5 immunization of human volunteers. Importantly, both vaccine-elicited antigen-specific CD4+ T cells and the total CD4+ T-cell population were transiently activated after the rAd5 immunization in the present nonhuman primate study.
Since the issue of vaccine-induced cell activation was raised in the wake of the findings of the STEP trial, attention has focused on rAd5-induced CD4+ T-cell activation as a consequence of vaccination. It is, however, important to know whether immunogen-induced T-cell activation occurs after administration of other vaccine modalities. In the present study we show that plasmid DNA vaccines do not activate CD4+ T cells and that rAd35 vaccines activate these cells, but less markedly than the rAd5 vaccines. These findings underscore the substantial differences between vectors in how they trigger T-cell responses and potential differences in their induction of global CD4+ T-cell activation.
Since the increased acquisition of HIV-1 in the STEP trial occurred in rAd5 vaccinated individuals who were Ad5 seropositive before vaccination (2, 10), it was important to assess immune activation after rAd5 administration in monkeys that were already Ad5 seropositive. Preexisting Ad5 immunity would best be generated by infecting monkeys with a wild-type, replication-competent adenovirus, since this would most closely model the acquisition of Ad5 immunity in humans. However, this could not be done because wild-type Ad5 does not replicate in rhesus monkeys (13). Although a host-range mutant of Ad5 has been created and might be used to model the induction of preexisting anti-Ad5 immunity in monkeys, we chose to use monkeys that received a prior inoculum of a replication defective rAd5 vaccine construct. Although this mode of inducing anti-Ad5 immunity was not optimal, the titers of anti-Ad5 antibodies in the serum of the monkeys used in these studies (range, 1,094 to 4,212) clearly approximates the titers of anti-Ad5 antibodies observed in African human populations.
rAd5 vaccination also induced a transient T-cell activation in monkeys with preexisting immunity to Ad5. Although no further expansion of the hexon-specific CD4+ and CD8+ T cells was detected after a second rAd5 immunization, both the magnitude and the polyfunctionality of the Gag-specific T-cell responses were boosted. These results therefore indicate that preexisting immunity to Ad5 blunted rAd5-induced hexon-specific T cells responses but had no effect on the Gag-specific T-cell responses.
It is interesting that the SIV Gag-specific CD8+ T cells and the hexon-specific CD8+ T cells responded differentially after repeated rAd5 immunization. The SIV Gag gene fragment in the rAd5 immunogen was cloned downstream of the cytomegalovirus promoter and was therefore potently expressed by the vaccine construct. In contrast to that, the rAd5 hexon gene was likely expressed at a much lower level in the replication-defective rAd5 vector with E1 deleted. Therefore, the first rAd5 boost induced a higher frequency of SIV Gag-specific CD8+ T-cell responses than of Ad5 hexon-specific CD8+ T-cell responses. After the second rAd5 immunization, preexisting Ad5-specific NAbs may have partially neutralized the rAd5 vaccine vector, resulting in a lower effective dose of the vaccine. This lower effective dose of the second rAd5 immunization may have further boosted the Gag-specific CD8+ T-cell responses but may have had a limited effect on the hexon-specific CD8+ T-cell responses.
HIV transmission occurs most often across mucosal barriers and CD4+ T cells are among the first cells infected during transmission (4). Activation of mucosal populations of T lymphocytes as a consequence of vaccination could certainly contribute to increasing the incidence of HIV transmission. To explore this possibility, we evaluated the kinetics of the activation status of total and antigen-specific mucosal T cells after rAd5 immunization in monkeys with preexisting immunity to Ad5. We found that parenteral rAd5 immunization induced lower-frequency SIV epitope-specific CD8+ T-cell responses and a delayed but more sustained total CD4+ and CD8+ T-cell activation in the colonic mucosa than in the peripheral blood. Such a sustained CD4+ T-cell activation may increase susceptibility to HIV transmission across a mucosal barrier. These results highlight the potential importance of evaluating mucosal T-cell populations in HIV-1 vaccine studies.
Acknowledgments
We are grateful to Adam P. Buzby, Angela Carville, and Michelle Lifton for technical assistance.
This study was supported in part by funds from the intramural research program of the Vaccine Research Center, National Institute of Allergy and Infectious Diseases, National Institutes of Health; Harvard Medical School CFAR grant AI060354; and CAVD CTC-VIMC grant 38650 from the Bill and Melinda Gates Foundation.
Footnotes
Published ahead of print on 5 August 2009.
REFERENCES
- 1.Betts, M. R., M. C. Nason, S. M. West, S. C. De Rosa, S. A. Migueles, J. Abraham, M. M. Lederman, J. M. Benito, P. A. Goepfert, M. Connors, M. Roederer, and R. A. Koup. 2006. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 107:4781-4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buchbinder, S. P., D. V. Mehrotra, A. Duerr, D. W. Fitzgerald, R. Mogg, D. Li, P. B. Gilbert, J. R. Lama, M. Marmor, C. Del Rio, M. J. McElrath, D. R. Casimiro, K. M. Gottesdiener, J. A. Chodakewitz, L. Corey, and M. N. Robertson. 2008. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 372:1881-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cayabyab, M. J., B. Korioth-Schmitz, Y. Sun, A. Carville, H. Balachandran, A. Miura, K. R. Carlson, A. P. Buzby, B. F. Haynes, W. R. Jacobs, and N. L. Letvin. 2009. Recombinant Mycobacterium bovis BCG prime-recombinant adenovirus boost vaccination in rhesus monkeys elicits robust polyfunctional simian immunodeficiency virus-specific T-cell responses. J. Virol. 83:5505-5513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haynes, B. F., and R. J. Shattock. 2008. Critical issues in mucosal immunity for HIV-1 vaccine development. J. Allergy Clin. Immunol. 122:3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koup, R. A., L. Lamoreaux, D. Zarkowsky, R. T. Bailer, C. R. King, J. G. Gall, D. E. Brough, B. S. Graham, and M. Roederer. 2009. Replication-defective adenovirus vectors with multiple deletions do not induce measurable vector-specific T cells in human trials. J. Virol. 83:6318-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letvin, N. L., Y. Huang, B. K. Chakrabarti, L. Xu, M. S. Seaman, K. Beaudry, B. Korioth-Schmitz, F. Yu, D. Rohne, K. L. Martin, A. Miura, W. P. Kong, Z. Y. Yang, R. S. Gelman, O. G. Golubeva, D. C. Montefiori, J. R. Mascola, and G. J. Nabel. 2004. Heterologous envelope immunogens contribute to AIDS vaccine protection in rhesus monkeys. J. Virol. 78:7490-7497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Letvin, N. L., J. R. Mascola, Y. Sun, D. A. Gorgone, A. P. Buzby, L. Xu, Z. Y. Yang, B. Chakrabarti, S. S. Rao, J. E. Schmitz, D. C. Montefiori, B. R. Barker, F. L. Bookstein, and G. J. Nabel. 2006. Preserved CD4+ central memory T cells and survival in vaccinated SIV-challenged monkeys. Science 312:1530-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McElrath, M. J. 2008. Vaccine-induced immunity in T-cell based candidate HIV vaccines, abstr. PL 01-01. AIDS Vaccine 2008, Cape Town, South Africa.
- 10.McElrath, M. J., S. C. De Rosa, Z. Moodie, S. Dubey, L. Kierstead, H. Janes, O. D. Defawe, D. K. Carter, J. Hural, R. Akondy, S. P. Buchbinder, M. N. Robertson, D. V. Mehrotra, S. G. Self, L. Corey, J. W. Shiver, and D. R. Casimiro. 2008. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet 372:1894-1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Brien, K. L., J. L., S. L. King, Y.-H. Sun, J. E. Schmitz, M. A. Lifton, N. A. Hutnick, M. R. Betts, S. A. Dubey, J. Goudsmit, J. W. Shiver, M. N. Robertson, D. R. Casimiro, and D. H. Barouch. 20 July 2009. Adenovirus-specific immunity following immunization with an Ad5 HIV-1 vaccine candidate in humans. Nat. Med. 15:873-875. [Epub ahead of print.] [DOI] [PMC free article] [PubMed]
- 12.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 13.Shiver, J. W., and E. A. Emini. 2004. Recent advances in the development of HIV-1 vaccines using replication-incompetent adenovirus vectors. Annu. Rev. Med. 55:355-372. [DOI] [PubMed] [Google Scholar]
- 14.Sprangers, M. C., W. Lakhai, W. Koudstaal, M. Verhoeven, B. F. Koel, R. Vogels, J. Goudsmit, M. J. Havenga, and S. Kostense. 2003. Quantifying adenovirus-neutralizing antibodies by luciferase transgene detection: addressing preexisting immunity to vaccine and gene therapy vectors. J. Clin. Microbiol. 41:5046-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun, Y., S. Santra, J. E. Schmitz, M. Roederer, and N. L. Letvin. 2008. Magnitude and quality of vaccine-elicited T-cell responses in the control of immunodeficiency virus replication in rhesus monkeys. J. Virol. 82:8812-8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun, Y., J. E. Schmitz, A. P. Buzby, B. R. Barker, S. S. Rao, L. Xu, Z. Y. Yang, J. R. Mascola, G. J. Nabel, and N. L. Letvin. 2006. Virus-specific cellular immune correlates of survival in vaccinated monkeys after simian immunodeficiency virus challenge. J. Virol. 80:10950-10956. [DOI] [PMC free article] [PubMed] [Google Scholar]