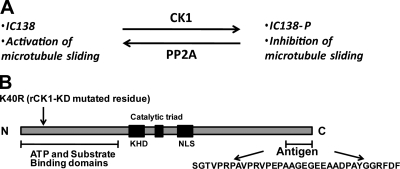
Figure 1.
Model for regulation of I1 dynein and the CK1 protein. (A) Analysis of wild-type and mutant axonemes has revealed that microtubule sliding activity is regulated by phosphorylation of the I1 dynein subunit IC138 (Wirschell et al., 2007). The data predicts that IC138 is phosphorylated by the axonemal kinase CK1, and that phosphorylation inhibits dynein-driven microtubule sliding activity. The model also indicates that axonemal phosphatase PP2A is required to rescue microtubule sliding activity (Yang and Sale, 2000). (B) C. reinhardtii CK1 is highly conserved and contains characteristic CK1 domains including the N-terminal ATP and substrate-binding domains, the kinesin homology domain (KHD), the catalytic triad, and the nuclear localization signal (NLS). To generate rCK1-KD, K 40, shown to be required for kinase activity (Gao et al., 2002), was replaced by R. A CK1-specific antibody was made to the polypeptide at the C terminus.