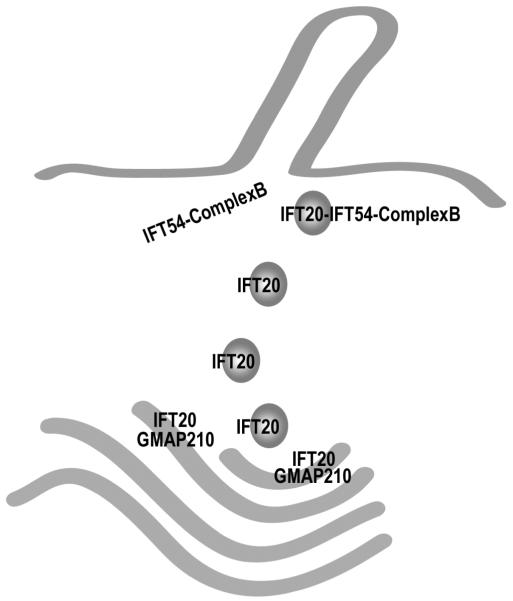
Figure 6. Model for the coordination of IFT20 in the Golgi IFT complex and complex B.
The finding that IFT20 is part of the Golgi IFT complex as well as complex B presents interesting possibilities for its function. This speculative model presents our view of how this may be occurring. IFT20 is anchored to the Golgi membrane by the golgin protein GMAP210 [Follit et al., 2008]. IFT20 is dynamic and moves from the Golgi complex to the cilium [Follit et al., 2006] but GMAP210 appears to remain at the Golgi. IFT20 binds directly to the complex B subunit IFT54 [this work and Omori et al. 2008] but IFT54 does not appear to localize to the Golgi complex and it is not part of the Golgi IFT complex [this work]. Thus, we hypothesize that IFT20 and GMAP210 function at the Golgi complex to sort proteins into vesicles destined for the ciliary membrane. These vesicles leave the Golgi with IFT20 associated with them. Once the vesicles arrive at the base of the cilium, IFT20 on the vesicles interacts with the IFT54 subunit of complex B to form the complete IFT complex. The vesicles fuse with the plasma membrane at the base of the cilium and the IFT complex with attached cargos is carried into the cilium by kinesin-2. By having the IFT complex assembled on the surface of the vesicle, the transport of membrane and non-membranous cargos could be coordinated.