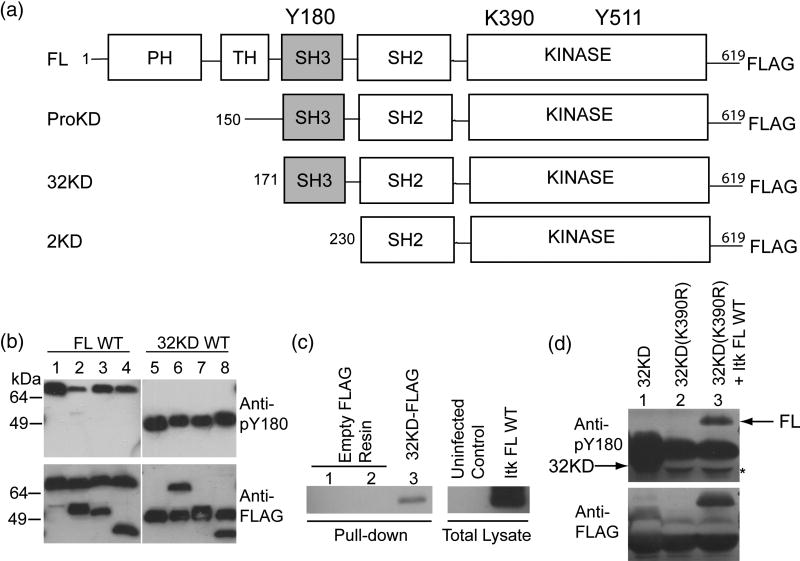
Figure 1. Itk autophosphorylation occurs in cis.
(A) Itk deletion fragments used in this study. Y180 in the SH3 domain is the site of autophosphorylation, K390 is mutated to R to render the kinase domain inactive, Y511 is the site of trans phosphorylation by Lck. (B) 0.5 μM Itk FL WT was incubated with 0.5 μM kinase inactive (K390R) versions of the following proteins: Itk ProKD (lane 2), Itk 32KD (lane 3) or Itk 2KD (lane 4), in a kinase assay buffer. Lane 1 is 1μM Itk FL WT alone. The samples were boiled, separated by SDS-PAGE and western blotted using an anti-phospho-Y180 or anti-FLAG antibody. Lanes 5–8: Reversal of the incubation scheme: Itk 32KD WT was incubated with kinase inactive (K390R) versions of the following proteins: Itk FL (lane 6), Itk ProKD (lane 7) and Itk 2KD (lane 8). Lane 5 is 1 μM Itk 32KD WT enzyme alone. The Itk 2KD fragment that lacks the Itk SH3 domain (lanes 4 and 8) is used as a control. (C) Itk FL can associate with Itk 32KD. Empty FLAG-resin (lanes 1 and 2) or purified FLAG-tagged Itk 32KD immobilized on an anti-FLAG resin (lane 3) was incubated with uninfected Sf9 cell lysate (lane 1) or untagged Itk FL infected Sf9 cell lysate (lanes 2 and 3) in a pull-down assay. The samples were rinsed, separated by SDS-PAGE and western blotted using an anti-Itk antibody. (D) In vivo Itk autophosphorylation occurs in cis. FLAG-tagged Itk 32KD WT, Itk kinase inactive 32KD (K390R) and Itk FL WT were co-expressed with Lck and immunoprecipitated from Sf9 cells using an anti-FLAG resin and western blotted as before with anti-phospho-Y180 or anti-FLAG antibodies. Lane 1: Itk 32KD WT alone, Lane 2: Itk kinase inactive 32KD (K390R) alone and Lane 3: Itk kinase inactive 32KD (K390R) co-expressed with Itk FL WT. Arrows on the top panel indicate the positions of Y180 phosphorylated Itk FL and Itk 32KD. Itk 32KD migrates slightly below the immunoglobulin heavy chain and a mobility shift associated with the phosphorylated Itk 32KD fragment is evident in lane 1 (the band is seen both below and slightly above the heavychain). The asterisk on the top panel indicates the heavy chain that has been displaced by the presence of Itk 32KD.