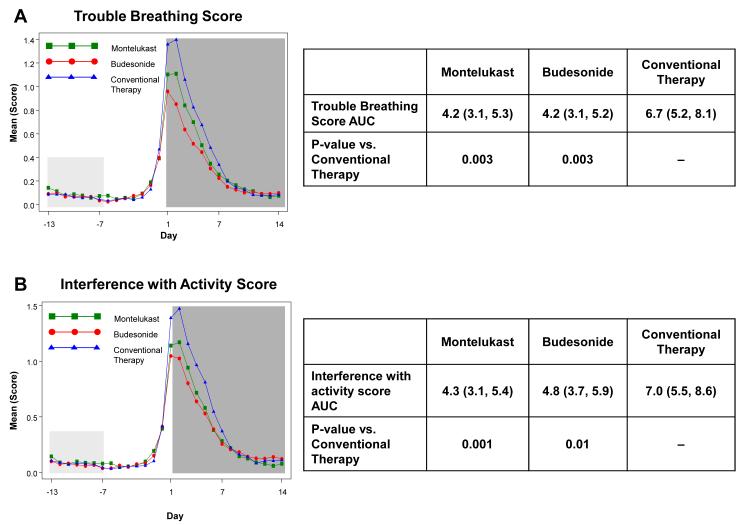
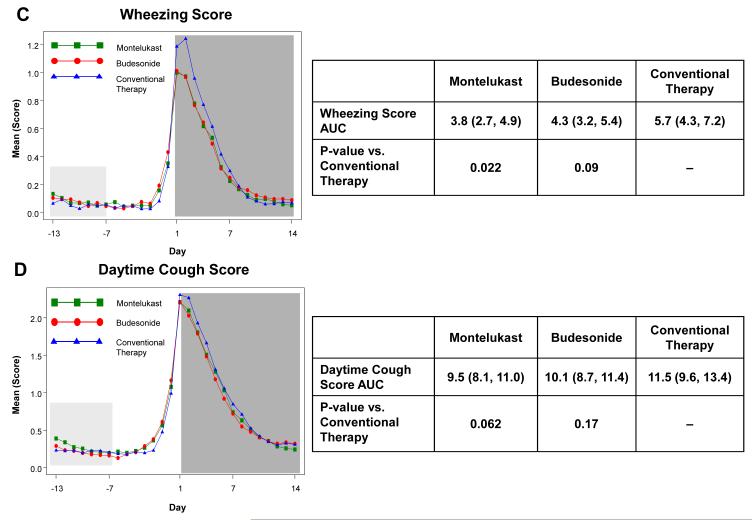
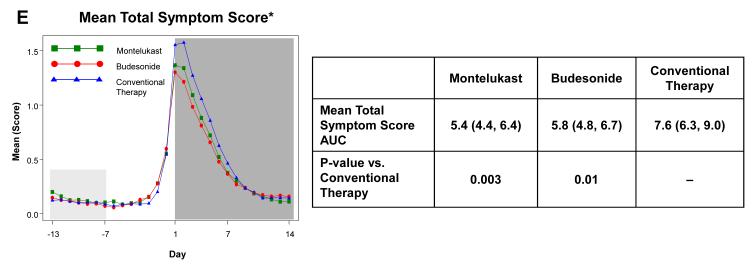
Figure 2. Area Under the Curve (AUC) during respiratory tract illnesses.
Area under the curve was calculated for the 14 days following initiation of study medication (shaded in dark grey) for symptoms scores of trouble breathing score (Panel A), interference with activity score (Panel B), wheezing (Panel C), daytime cough (Panel D), and mean total symptom score (Panel E). This value was analyzed as a difference from ‘baseline’ symptom levels, defining baseline as twice the AUC from Days −13 to −7, which preceded onset of symptoms (shaded in light grey). The tables present the AUCs (95% CIs) and p-values comparing each active therapy to conventional therapy. There were no significant differences between montelukast and budesonide for any of these symptom measures (p>0.4).
* Mean of Daytime Cough + Wheezing + Trouble Breathing + Interference with Activity