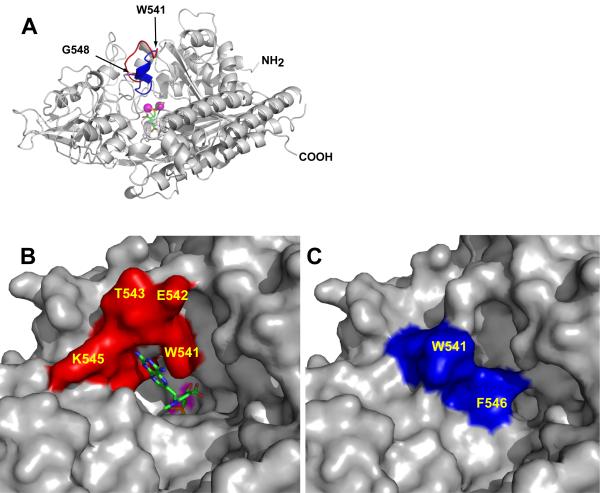
Figure 2. The substrate binding cavity of GCPII can be closed by the ‘entrance lid’.
Panel A, Superposition of the ‘entrance lids’ from rhGCPII/MPE and rhGCPII/SPE complexes. The model of rhGCPII/MPE is shown in cartoon representation and colored gray. The ‘entrance lid’, formed by the amino acids Trp541-Gly548, is painted in red and blue for the open (observed in the rhGCPII/MPE complex) and closed (taken from the superimposed structure of the rhGCPII/SPE complex) conformations, respectively. The active site-bound SPE inhibitor is represented by sticks and the active-site Zn2+ ions by magenta spheres. Panels B and C, A close-up view of the ‘entrance lid’ in open/closed conformation. The protein is represented by its molecular surface with the ‘entrance lid’ colored in red or in blue for the open (rhGCPII/MPE, Panel B) and closed (rhGCPII/SPE, Panel C) conformations, respectively. The molecule of MPE, visible in the current projection, is represented by sticks (Panel B), while a molecule of SPE is buried by the ‘entrance lid’ (Panel C). A surface defined by the active-site zinc ions is colored in magenta.