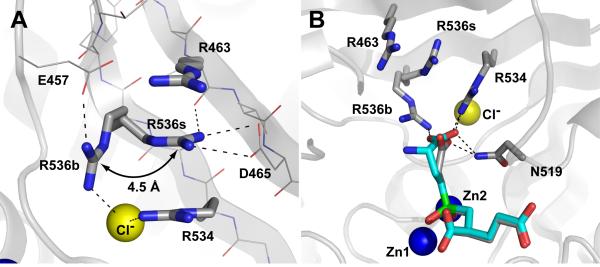
Figure 5. The side chain of Arg536 adopts two alternate conformations.
Panel A, Arrangement of S1 arginines in the rhGCPII/SPE complex. Arginine 536 can adopt two distinct conformations referred to as the ‘stacking’ (R536s) and ‘binding’ (R536b) conformation, respectively. Transition between the two conformations is associated with a 4.5 Å shift of the guanidinium group. The atoms are colored blue (nitrogen), red (oxygen), gray (carbon) and yellow (the chloride ion). Polar interactions stabilizing individual conformations of Arg536 are shown as dashed lines. The SPE inhibitor and water molecules have been omited for clarity. Panel B, Superposition of SPE and EPE inhibitors in the substrate-binding cavity of GCPII. The rhGCPII/EPE and rhGCPII/SPE complexes were superimposed based on corresponding Cα atoms (only GCPII atoms from the rhGCPII/SPE complex are shown). The S1 residues and the active-site bound inhibitors are shown in stick representation, Zn2+ and Cl- ions as blue and yellow spheres, respectively. The atoms are colored blue (nitrogen), red (oxygen), gray (rhGCPII/SPE carbons), cyan (EPE carbons), and green (phosphorus). Direct polar interactions between inhibitors and the protein in the S1 pocket are shown as dashed lines.