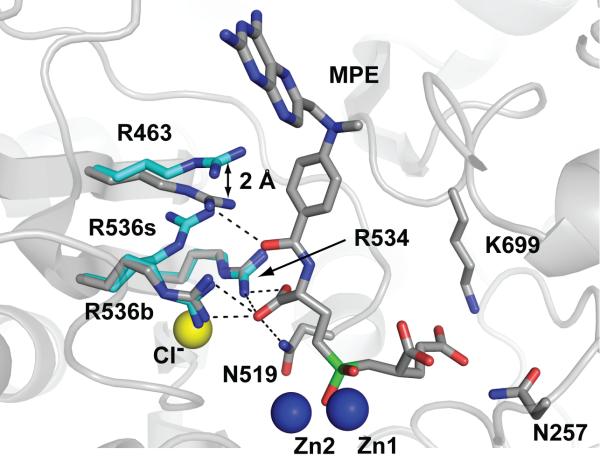
Figure 6. MPE binding leads to rearrangement of the S1 arginines of GCPII.
Repositioning of Arg463 by 2.0 Å prevents Arg536 from adopting the ‘stacking’ (R536s) conformation. As a result, only the ‘binding’ (R536b) conformation of Arg536 is observed in the rhGCPII/MPE complex. The rhGCPII/MPE and rhGCPII/EPE complexes were superimposed using corresponding Cα atoms. A protein part of the rhGCPII/MPE complex is shown in cartoon representation and selected amino acid residues from both complexes and the active-site bound MPE are shown in stick representation. Zn2+ and Cl- ions are depicted as blue and yellow spheres, respectively. Atoms are colored blue (nitrogen), red (oxygen), gray (rhGCPII/MPE carbons), cyan (rhGCPII/EPE carbons) and green (phosphorus). Direct H-bonding interactions between MPE and the protein in the S1 pocket are shown as dashed lines.