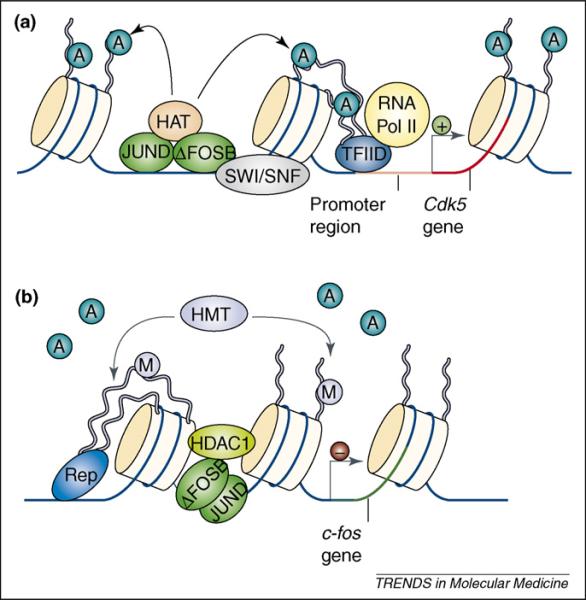
Figure 3.
Gene-dependent recruitment of chromatin-remodeling enzymes. (a) Cocaine- and amphetamine-induced increases in ΔFosB in the nucleus accumbens (NAc) are known to activate transcription of the cdk5 gene. This involves binding of the ΔFosB/JunD heterodimer to the cdk5 promoter and recruitment of the SWI/SNF ATP-dependent chromatin-remodeling complexes and histone acetyltransferases (HATs). This allows for the assembly of RNA polymerase II (RNA Pol II), Transcription Factor IID (TFIID) and several other protein complexes (not shown) involved in the initiation and elongation of transcription. Histone deacetylases (HDACs) are not present on the cdk5 promoter, which permits significantly higher levels of acetylated histone H3 after chronic cocaine exposure. (b) Cocaine- and amphetamine-induced increases in ΔFosB also act as a transcriptional repressor at a different gene locus, c-fos. After repeated stimulant exposure, the c-fos gene is desensitized in the NAc and much more weakly induced by subsequent drug exposures. This involves the binding of ΔFosB to the c-fos gene promoter and recruitment of HDAC1 to reduce histone acetylation and gene activity. In concert with HDAC1, chronic drug exposure increases the levels of the repressive histone methyltransferase (HMT); KMT1a/SUV39H1 and levels of histone H3K9 methylation on the c-fos promoter. Together, these enzymes and histone modifications serve to repress c-fos gene activity through a mechanism involving ΔFosB and other corepressors (Rep). Figure modified with permission from [24].