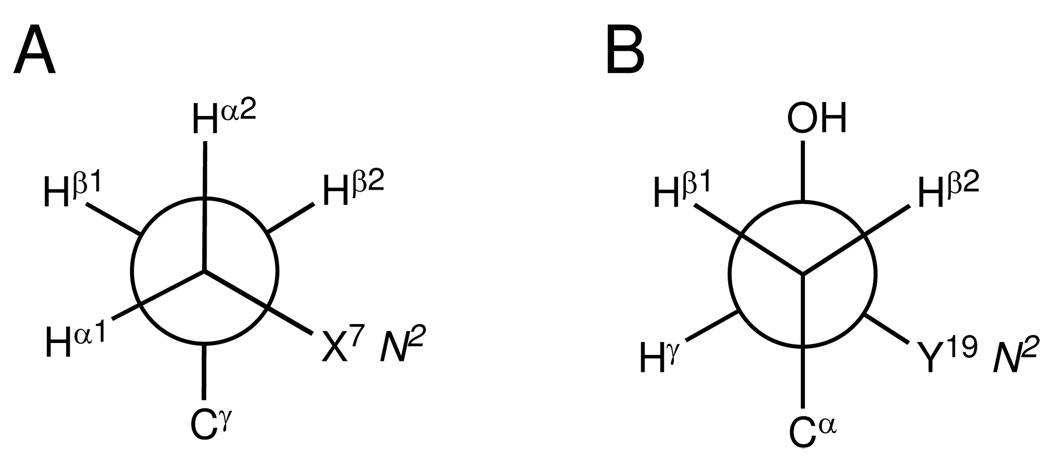
Figure 7.
Newman projections showing conformation of the (R)-γ-hydroxytrimethylene tether. (A) Viewed along Cα-Cβ bond. (B) Viewed along Cβ-Cγ bond. Both X7 N2 and Y19 N2 were in the gauche-conformation with the tether to maintain the Watson-Crick hydrogen bonding. The trans-conformation of hydroxyl group with Cα minimizes the steric interaction and allows the formation of the carbinol OH→C20 O2 hydrogen bond.