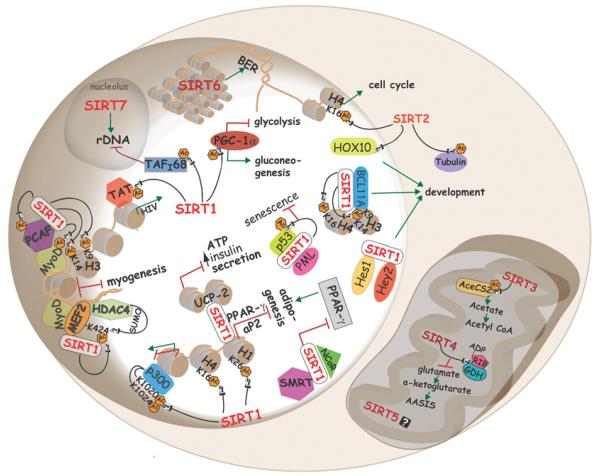
Figure 4. Cellular functions of mammalian sirtuins.
Sirtuins regulate a variety of process in mammalian cells. For example, in the nucleus, SIRT1 modulates chromatin structure by deacetylating specific lysine residues in histones H1, H3 and H4. Also, SIRT1 alters gene expression by targeting Lys1020 and Lys1024 of p300. Deacetylation of Tat by SIRT1 promotes HIV-1 replication. SIRT1 complexes with PML and mediates p53 deacetylation, thus protecting cells from senescence. SIRT1 represses muscle differentiation by two different mechanisms that involve MyoD: (i) in co-operation with PCAF and (ii) by deacetylating Lys424 of MEF2, which promotes its sumoylation by HDAC4, leading to suppression of myogenic genes. SIRT1 interacts with PPAR-γ and aP2 promoters, as well as with the PPAR-γ cofactors NcoR and SMRT to inhibit adipogenesis. Interactions of SIRT1 with BCL11A or Hes1/Hey2, and SIRT2 with the homeobox transcription factor, HOXA10, may regulate development. Glucose metabolism and energy modulated through deacetylation of PCG-1α and transcriptional repression of UCP2 respectively. SIRT6 mainly localizes to heterochromatin to regulate base excision repair, and SIRT2 shuffles from the cytoplasm to the nucleus during mitosis, where it deacetylates Lys16 of histone H4. In the nucleolus, SIRT7 activates RNA Pol I transcription, whereas SIRT1 represses it by deacetylating TAFI68. Cytoplasmic SIRT1 and mitochondrial SIRT3 deacetylate and activate AceCS1 and AceCS2 respectively. SIRT4 mono-ADP-ribosylates GDH, which inhibits amino-acid-stimulated insulin secretion. AC, acetylation.