Abstract
Objective
In order to further our understanding of how intentional weight loss (IWL) and overeating are related, we examined the shared genetic and environmental variance between lifetime IWL and overeating.
Methods
Interview data were available for 1976 female twins (both members of 439 and 264 pairs of monozygotic and dizygotic twins respectively), mean age=40.61, SD=4.72. We used lifetime diagnostic data for eating disorders obtained from a semi-structured psychiatric telephone interview, examined in a bivariate twin analysis. Both lifetime behaviours were measured on a 3-point scale, where absence of IWL or overeating formed one anchor on the scale and lifetime anorexia nervosa (AN) and bulimia nervosa (BN) formed the opposite anchors respectively.
Results
In line with previous findings, a higher body mass index was significantly associated with the lifetime presence of IWL and/or overeating (odds ratio=1.13, 95% confidence interval (CI): 1.08–1.19). The best fitting twin model contained additive genetic and non-shared environmental influence influencing both IWL and overeating, with correlations between these influences of 0.61 (95% CI: 0.35–0.92) and 0.24 (95% CI: 0.07–0.42) respectively.
Conclusion
About 37% of genetic risk factors were considered to overlap between IWL and overeating, and with only 6% of overlap between environmental risk factors. Thus considerable independence of risk factors was indicated.
Both intentional weight loss (IWL) and overeating (eating large amounts of food in a short period of time) are components of eating disorder criteria but the exact nature of the relationship between the two phenotypes is unclear. Whilst ostensibly appearing to be at opposite ends of the behavioural spectrum, we know that both young and mid-aged adults who engage in IWL are significantly more likely to overeat1 and that overeating is strongly associated with strict dieting2. Therefore, on the one hand, IWL and overeating do appear closely related. Additionally, eating disorders that are primarily characterized by IWL and overeating seem closely related. For example, lifetime eating examined in a latent profile analysis of a community sample of twins resulted in five profiles, only one of which contained women with clinically significant lifetime eating disorders, including disorders related primarily to either weight loss or overeating3.
A better understanding of the relationship between IWL and overeating would also inform our understanding of the relationship between anorexia nervosa (AN) and bulimia nervosa (BN), even though these disorders are defined by more extreme versions of these behaviours, namely obtaining underweight and objective binge episodes (eating large amounts of food in a short period of time accompanied by feeling out of control), respectively. Over thirty years ago BN was described as “an ominous variant” of AN4, in part driven by the observation that many patients with BN reported a history of AN. Subsequent research tells us that between 22% and 54% of women with AN go on to develop BN5,6, where cross-diagnostic similarities (e.g., binge eating, extreme dietary restraint) become more marked over a lifetime perspective7, and a common familial vulnerability between the two disorders exists8. On the other hand, it has been suggested that eating disorders involving binge eating and overweight are discontinuous with normalcy and differ in nature from eating disorders involving weight loss as these latter disorders may be dimensional in character9. While there is some overlap between the risk factor profiles for eating disorders that are primarily differentiated by IWL or overeating, there are many risk factors that are specific to each eating disorder10–12.
These two different stances can be somewhat reconciled by the hypothesis that the diathesis-stress model is of relevance to the similarities and differences observed between IWL and overeating, where it can be suggested that the genetic risk factors for the two behaviours are largely similar between the two behaviours, but that environmental risk factors that trigger the this genetic susceptibility are different, thus resulting in different features of eating being expressed. For example, high parental expectations have been more strongly implicated with BN than AN onset12–13. Hence the current investigation sought to examine shared genetic and environmental factors between eating that involved IWL and overeating in a large adult female twin sample.
Method
Participants
Participants were from the volunteer adult Australian Twin Registry (ATR) formed and maintained by the National Health and Medical Research Council. These data are from women who participated in telephone interviews over 1992–93, (N=3848, aged 27–90 years). These twins had previously participated in a mailed questionnaire survey over 1980–82 and a follow-up questionnaire survey over 1988–90 where they self-reported their height, and current, maximum and minimum weight. From those pairs where at least one twin had responded to the second survey, interviews were completed with 3659 eligible women (88.3%), and excluding those who were deceased, overseas, not locatable, or who had previously withdrawn from the ATR, the response rate for women from this sample was 92.3%14. Given that it is not uncommon for disordered eating to onset in the mid-20s15, and given the problems identified with longer–term recall of psychiatric history16, only women aged over 30 and under 50 years were selected for inclusion in the current study. This was to allow time for experience with eating problems to occur and to increase reliability of recall. There were 1976 women who completed the interview (mean age of 40.61 years, SD=4.72), including both members of 703 twin pairs, 439 monozygotic (MZ) and 264 dizygotic (DZ). Females in the ATR sample are largely representative of the general Australian female population on a variety of indicators including age, general level of education, and marital status17.
Zygosity was determined blindly by standard questions that have >95% accuracy18. More recently, members of a subsample of 198 same-sex pairs from this group, who reported they were MZ, were typed for 11 independent highly polymorphic markers in the course of an asthma study19. No errors in our previous zygosity diagnosis were detected.
Each twin had previously signed a consent form to be approached for scientific studies and verbal assent was obtained prior to telephone interviews. All applicable institutional regulations concerning the ethical use of human volunteers were followed during this research.
Delineation of the IWL and overeating phenotypes
The psychiatric interview utilised was the Semi-Structured Assessment for the Genetics of Alcoholism, modified for use in Australia20, an interview format that includes skip rules. It comprises items previously validated by other research interviews, such as the Composite International Diagnostic Interview (CIDI)21. A subset of these questions assessed the lifetime presence of DSM-IV AN and BN. These questions, and the order in which they were asked, is shown in Table 1. As us typical in such large interview schedules, skip rules were used, such that negative responses resulted in the interviewer moving on to the next diagnostic section of the interview. Responses to the interview questions for each diagnosis were divided into three categories. In the case of the IWL phenotype, the first category included only those women who met criteria for AN (with or without amenorrhea; N=22, 1.4%), the second category included women who had lost a lot of weight on purpose or kept their weight down on purpose (i.e., answered “yes” to only the first intentional weight loss question), but did not meet any other criteria for AN (N=429, 27.0%), and the third and largest group included women who answered “no” to the first intentional weight loss question and thus were not asked any further of the diagnostic questions for AN (N=1133, 71.6%). In the case of eating disorders that involved overeating, the first category included only those women who met full criteria for BN and thus experienced objective binge episodes (N=23, 1.4%), the second category included women who had experienced overeating (i.e., answered “yes” to at least the first two of the overeating questions) but did not necessarily experience loss of control and definitely did not use weight control behaviours (N=232, 13.9%), and the third group had never experienced any lifetime overeating (N=1416, 84.7%). There was no overlap between the women who reported AN and BN, but 94 women reported IWL and overeating, and 1033 women reported neither behaviour.
Table 1.
Interview questions asked to obtain phenotype measures
| Intentional Weight Loss | Overeating |
|---|---|
| Did you ever lose a lot of weight on purpose, or, while you were growing up, did you keep your weight down on purpose? | Were you ever greatly concerned about eating too much, looking too fat, or gaining too much weight? |
| Did you ever feel fat, even though your family and friends were very concerned that you had become much too thin? | Has there ever been a time in your life when you went on eating binges – eating a large amount of food in a short period of time (usually less than 2 hours)? |
| After purposely losing a lot of weight, what is the lowest weight you ever dropped to? Did friends say you were to thin or skeleton like? | Did you go on eating binges as often as twice a week? |
| How tall were you at the time? How old were you? | During these binges were you afraid that you could not stop eating, or that your eating was out of control? |
| Were you intensely afraid of gaining weight or becoming fat? While you were losing weight did your period stop for 3 cycles or more (when you were not pregnant)? | Did you do anything to prevent weight gain from binge eating, such as making yourself vomit, taking laxatives or diuretics, dieting strictly, fasting, exercising vigorously, or anything else? |
Statistical Analyses
In order to describe phenotype membership by body mass index (BMI), the three groups within each phenotype were compared using linear mixed-effects modeling in SPSS (fixed-effects models with non-residual errors). As the twin data contains correlated observations and the assumption of independent sampling was violated, this approach corrects the p value accordingly. Examination of the relationship between BMI and the presence of either lifetime IWL and/or overeating was conducted using generalized estimating equations, which also corrected for correlated observations. Bonferroni corrected adjustments were used to evaluate all post-hoc comparisons.
The lifetime data pertaining to the IWL and overeating phenotypes were examined in a bivariate twin analysis. Given that the data was negatively skewed, the normal weights of the scores were used (i.e., using the liability threshold model). We used Mx22 to fit a bivariate genetic Cholesky decomposition model using the weighted least squares method, with the input data in the form of twin pair polychoric correlation matrices and the associated asymptotic covariance matrices, generated using PRELIS223. The population variance of each variable can be due to three different influences: additive genes (A), common or shared environment (C), and non-shared or unique environment (E). In a bivariate model, the correlation between the two phenotypes can be divided into that due to these three different influences, ra (the degree to which genetic sources of variance for the two phenotypes overlap), rc (the degree to which common environmental sources of variance for the two phenotypes overlap), or re (the degree to which non-shared sources of environmental variance for the two phenotypes overlap). Initially, a full model (ACE and the correlations between all three parameters) was fit to the data. Subsequently increasingly restrictive models were compared to the fit of this full model. The goal of model fitting is to explain the observed data as an optimal combination of goodness-of-fit and parsimony. Akaike’s Information Criterion (AIC24) reflects these criteria, where the lower (or more negative) the value, the better the fit of the model.
Results
Description of phenotype membership
In order to examine the relation between self-reported body mass index (BMI) and phenotype membership, the means of the current, maximum and minimum BMI were examined and are presented in Table 2. Within the intentional weight loss phenotype, it can be seen that the group with AN had a significantly lower current and minimum BMI than other groups, and that the second group, those women who has lost a lot of weight or sought to keep their weight down, had significantly higher BMI (current, maximum and minimum) than the other two groups, suggesting that these were women who battled overweight and used intentional weight loss as a weight control strategy.
Table 2.
Comparison of the current, maximum and minimum body mass index (BMI) for the intentional weight loss and the overeating phenotypes
| BMI variable | Intentional Weight Loss |
F | Overeating |
F | ||||
|---|---|---|---|---|---|---|---|---|
| anorexia nervosa (n=22) | lost a lot of weight/kept it down (n=429) | no weight loss (n=1133) | p | bulimia nervosa (n=23) | overeating only (n=232) | no overeating (n=1416) | p | |
| BMI - current | 20.59 (2.43) 1 | 24.35 (4.52) 2 | 22.57 (3.79) 3 | 18.57 | 25.90 (4.67) 1 | 24.19 (4.83) 1 | 22.76 (3.84) 2 | 7.60 |
| <0.001 | 0.001 | |||||||
| BMI - maximum | 23.71 (3.45) 1 | 26.72 (5.12) 2 | 24.04 (3.99) 1 | 32.16 | 29.21 (4.85) 1 | 26.53 (5.67) 1 | 24.39 (4.08) 2 | 18.48 |
| <0.001 | <0.001 | |||||||
| BMI- minimum | 17.61 (2.17) 1 | 20.51 (2.26) 2 | 19.44 (2.27) 3 | 31.76 | 21.15 (2.39) 1 | 20.17 (2.45) 1 | 19.59 (2.26) 2 | 4.44 |
| <0.001 | 0.012 | |||||||
Note: Superscripts indicate which groups differ within each of the two phenotypes
Within the overeating phenotype, there was no difference in BMI between the women with BN and the women who reported overeating, but both groups had a significantly higher BMI (current, maximum and minimum) than the group of women who reported never overeating.
Across the two phenotypes, the presence of lifetime overeating or IWL was significantly associated with a higher BMI (odds ratio (OR)=1.13, 95% confidence interval (CI): 1.08–1.19), higher maximum BMI (OR=1.16, 95% CI: 1.11–1.22) and higher minimum BMI (OR=1.22, 95% CI: 1.12–1.33).
Cross-twin and cross-trait correlations
The correlations between IWL and overeating for Twin 1 and Twin 2 are shown in Table 3. These behaviours were positively correlated within each twin (+0.196 and +0.355 for MZ twins and +0.401 and +0.517 for DZ twins). Cross-twin IWL was correlated at +0.297 and +0.119 for MZ and DZ twins respectively, and cross-twin overeating was correlated at +0.422 and +0.071 for MZ and DZ twins respectively. These cross-twin correlations suggest a role for genetic contribution to both the behaviours. Across the twins in each pair, IWL and overeating correlated between 0.206–0.276 for MZ twins, and between −0.196 and 0.139 for DZ twins, indicating that ra would be associated with larger correlations than re.
Table 3.
Cross-trait and cross-twin correlations for eating disorders that involved intentional weight loss (IWL) and overeating: MZ correlations are in the top right of the diagonal in italics and DZ correlations are in the bottom left of the diagonal.
| IWL Twin 1 | Overeating Twin 1 | IWL Twin 2 | Overeating Twin 2 | |
|---|---|---|---|---|
| IWL Twin 1 | 1.00 | 0.196 | 0.297 | 0.276 |
| Overeating Twin 1 | 0.401 | 1.00 | 0.206 | 0.422 |
| IWL Twin 2 | 0.119 | −0.196 | 1.00 | 0.355 |
| Overeating Twin 2 | 0.139 | 0.071 | 0.517 | 1.00 |
Shared genetic and environmental risk factors
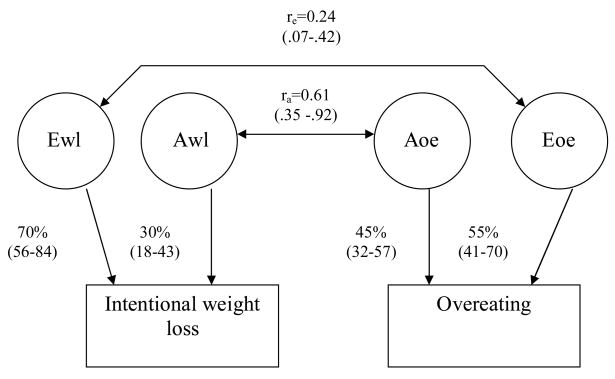
In addition to the full model in the bivariate model fitting, we tested ten nested sub-models. Only three sub-models did not offer a significantly worse fit than the full model (reported in Table 4), namely models 2, 5 and 6. The best-fitting and most parsimonious model, determined by AIC values, was the AE rare sub-model (model 5), depicted in Figure 1. In this model, the majority of the variance for IWL is accounted for by non-shared environmental factors (70%, which includes measurement error) with the remaining variance being accounted for by genetic variance. The variance for the overeating phenotype was less influenced by non-shared environmental factors, which accounted for 55% of the variance, with 45% being attributed to additive genetic influence. The proportion of shared genetic variance between the two behaviours was 37% (i.e., ra2) with 95% confidence intervals (CI) of 12% and 85%. The proportion of non-shared environmental variance common to the two behaviours (i.e., re2) was 6% (95% CI: 0.49%–18%). Any model setting a correlation between additive genetic factors of the two behaviours to zero was excluded, suggesting the presence of common genetic risk factors between the two phenotypes.
Table 4.
Fit statistics for bivariate twin analyses between lifetime intentional weight loss and overeating
| Model # | Model | χ2 fit (p) | df | χ2 diff (df) p | AIC |
|---|---|---|---|---|---|
| 1 | ACE rarcre | 17.96 (0.08) | 11 | −4.04 | |
| 2 | ACE rare | 19.58 (0.08) | 12 | 1.62 (1) >0.05 | −4.42 |
| 3 | ACE rcre | 26.27 (0.01) | 12 | 8.31 (1) <0.05 | 2.72 |
| 4 | ACE re | 39.89 (<0.001) | 13 | 21.93 (2) <0.05 | 13.89 |
| 5 | AE rare | 19.67 (0.14) | 14 | 1.71 (3) >0.05 | −8.34 |
| 6 | AE ra | 27.10 (0.03) | 15 | 9.14 (4) >0.05 | −2.90 |
| 7 | AE re | 39.91 (<0.001) | 15 | 21.95 (4) <0.05 | 9.91 |
| 8 | CE rcre | 29.07 (0.01) | 14 | 11.11 (3) <0.05 | 1.07 |
| 9 | CE rc | 43.83 (<0.001) | 15 | 25.87 (4) <0.05 | 13.83 |
| 10 | CE re | 43.81 (<0.001) | 15 | 25.85 (4) <0.05 | 13.81 |
| 11 | E re | 95.07 (<0.001) | 17 | 77.08 (6) <0.05 | 61.04 |
Note: Additive genetic variance (A), shared environmental influence (C) and non-shared environmental influence (E) are signified for both intentional weight loss and overeating, with the correlation term referring to the correlations between the latent factors contributing to the two phenotypes. Best fitting model in bold.
Figure 1.
The best-fitting and most parsimonious bivariate model examining the overlap between additive genetic (A) and non-shared environmental (E) risk factors for intentional weight loss and overeating (labeled Aiwl and Eiwl and Aoe and Eoe respectively). The pathways estimates (with 95% confidence intervals) represent the proportion of variance in the dependent variable accounted for by the predictor variable. The correlation between the two latent sources of variance is labeled ra for additive genetic influence and re for non shared environmental influence.
DISCUSSION
The purpose of the current research was to examine the hypothesis that genetic risk factors would be largely similar between IWL and overeating, but that environmental risk factors would be different. This finding would in part explain why there are both observed overlap and differences between these behaviours and eating disorders that are typified by these behaviours. Consistent with previous diagnostic studies of disordered eating25, our best fitting and most parsimonious bivariate model included only additive genetic and non-shared environmental influences for our two phenotypes, namely IWL and overeating. None of the cross-twin, cross-trait correlations for the two phenotypes strongly supported the contribution of the shared environment, and our model fitting suggested that the inclusion of additive genetic influence and the non-shared environment was necessary to the fit of the model.
Our estimation that 18%–43% of genetic variance contributed to the IWL phenotype was similar to the estimation of an IWL phenotype in a Finnish twin population1 for men (19%–55%) but lower than for women (55%–75%), and overlapped with the lower end of the range estimated for AN syndromes from a variety of twin populations: 33%–84% in the Virginia Twin Registry26, 0%–87% in a Swedish cohort of twins27, and 35%–95% in 17-year old twins from Minnesota28 (although this latter estimate is limited by the fact that most of the twins had not passed through the period of risk for eating disorder onset). Our phenotype involving overeating, where the contribution of genetic variance ranges from 32%–57%, was within the lower range of 31%–83% for BN suggested by a review of twin studies25, where the higher estimate was obtained for broadly defined BN when measurement error was diminished but reliability of diagnosis was low29.
Whilst our data did not support the hypothesis that genetic risk factors were largely similar between IWL and overeating, the hypothesis that there were more shared genetic factors than environmental risk factors between the two phenotypes was supported. Our best fitting model suggests that 37% of the genetic risk factors were shared between our two phenotypes, with non-zero 95% CI of 13% to 85%, and that only 6% of the individual-specific environmental risk factors are shared between behavioral phenotypes, also with nonzero 95% CI, ranging from 0.5% to 18%. Future research needs to consider unique sources of genetic variance that may contribute to the differential development of IWL and overeating, such as those genetic influences that are associated with temperament. Additionally, specific environments that influence the differential development of IWL and overeating require identification.
These results should be interpreted within the context of five important limitations. First, while our models indicate good fit, we lacked sufficient power to definitively choose only one model, with three different models not significantly worse fitting than the full model. However, these three models gave similar estimates and all supported the contribution of additive genetic and non-shared environment, with the latter having the greatest contribution to the variance of both phenotypes. Second, we used a highly reliable and valid psychiatric interview, where a previous comparison in this population of the agreement between the SSAGA and the Eating Disorder Examination30 for the diagnosis of BN has shown a kappa of 0.5931, the higher end for reporting of psychiatric diagnoses on two different occasions16. However, the retrospective reporting of psychiatric diagnoses is problematic, as reliability of lifetime reporting has been shown to be improved with increasing severity of the eating symptomatology32. Third, our community sample was drawn from a twin population, but to date no studies indicate any differences in rates of psychopathology compared to the general population33. Fourth, if eating involving weight loss is discontinuous with eating that is associated with binge eating or overweight9, then this would violate one of the assumptions of our bivariate modeling, namely the underlying normalcy of the phenotype. However, this suggestion of discontinuity requires further investigation before it can be accepted. Finally, there may be other explanations of shared genetic variance between the two behaviours and future research should examine the role of other phenotypes that are related to both IWL and overeating, such as specific types of temperament.
In summary, our answer to the question of whether IWL and overeating are closely related is that the two phenotypes are neither completely independent nor are they highly overlapping disorders. It appears that the causes of comorbidity between the two phenotypes may be primarily due to genetic influences. It is likely that environmental influences not shared between the twin pair may be important in influencing the differential development of one type of behaviour over another.
Acknowledgments
This research was supported by grants from the National Health and Medical Research Council (Australia) to N.G.M., National Institute of Alcoholism and Alcohol Abuse (AA 07535, AA 07728 and AA11988, USA) to A.C.H. We thank Dixie Statham for her co-ordination of Wave 2 interviews. We would also like to thank the twins for their participation in this research.
References
- 1.Keski-Rahkonen A, Neale BM, Bulik CM, Pietiläinen KH, Rose RJ, Kaprio J, Rissanen A. Intentional weight loss in young adults: Sex-specific genetic and environmental effects. Obesity Res. 2005;13:745–753. doi: 10.1038/oby.2005.84. [DOI] [PubMed] [Google Scholar]
- 2.Smith CF, Williamson DA, Bray GA, Ryan DH. Flexible vs. rigid dieting strategies: Relationship with adverse behavioral outcomes. Appetite. 1999;32:295–305. doi: 10.1006/appe.1998.0204. [DOI] [PubMed] [Google Scholar]
- 3.Wade TD, Crosby RD, Martin NG. Use of latent profile analysis to identify eating disorder phenotypes in an adult Australian twin cohort. Arch Gen Psychiatry. 2006;63:1377–1384. doi: 10.1001/archpsyc.63.12.1377. [DOI] [PubMed] [Google Scholar]
- 4.Russell G. Bulimia nervosa: An ominous variant of anorexia nervosa. Psychological Med. 1979;9:429–448. doi: 10.1017/s0033291700031974. [DOI] [PubMed] [Google Scholar]
- 5.Bulik CM, Sullivan PF, Fear J, Pickering A. Predictors of the development of bulimia nervosa in women with anorexia nervosa. J Nervous Mental Disease. 1997;185:704–707. doi: 10.1097/00005053-199711000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Braun D, Sunday S, Halmi K. Psychiatric co-morbidity in patients with eating disorders. Psychological Med. 1994;24:859–867. doi: 10.1017/s0033291700028956. [DOI] [PubMed] [Google Scholar]
- 7.Milos G, Spindler A, Schnyder U, Fairburn GG. Instability of the eating disorder diagnoses: A prospective study. Br J Psychiatry. 2005;187:573–578. doi: 10.1192/bjp.187.6.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lilenfeld L, Kaye W, Greeno C, Merikangas K, Plotnikov K, Pollice C, Rao R, Strober M, Bulik C, Nagy L. A controlled family study of restricting anorexia and bulimia nervosa: Comorbidity in probands and disorders in first-degree relatives. Arch Gen Psychiatry. 1998;55:603–610. doi: 10.1001/archpsyc.55.7.603. [DOI] [PubMed] [Google Scholar]
- 9.Williamson DA, Gleaves DH, Stewart TM. Categorical versus dimensional models of eating disorders: An examination of the evidence. Int J Eat Disord. 2005;31:1–10. doi: 10.1002/eat.20074. [DOI] [PubMed] [Google Scholar]
- 10.Fairburn CG, Welch SL, Doll HA, Davies BA, O’Connor ME. Risk factors for bulimia nervosa. A community-based case-control study. Arch Gen Psychiatry. 1997;54:509–517. doi: 10.1001/archpsyc.1997.01830180015003. [DOI] [PubMed] [Google Scholar]
- 11.Fairburn CG, Cooper Z, Doll HA, Welch SL. Risk factors for anorexia nervosa. Three integrated case-control comparisons. Arch Gen Psychiatry. 1999;56:468–476. doi: 10.1001/archpsyc.56.5.468. [DOI] [PubMed] [Google Scholar]
- 12.Jacobi C, Hayward C, de Zwaan M, Kraemer HC, Agras WS. Coming to terms with risk factors for eating disorders: Application of risk terminology and suggestions for a general taxonomy. Psychological Bull. 2004;130:19–65. doi: 10.1037/0033-2909.130.1.19. [DOI] [PubMed] [Google Scholar]
- 13.Wade TD, Gillespie N, Martin NG. A comparison of early family life events amongst monozygotic twin women with lifetime anorexia nervosa, bulimia nervosa or major depression. International Journal of Eating Disorders. 2007;40:679–686. doi: 10.1002/eat.20461. [DOI] [PubMed] [Google Scholar]
- 14.Heath AC, Bucholz KK, Madden PAF, Dinwiddie SH, Slutske WS, Bierut LJ, Statham DJ, Dunne MP, Whitfiled JB, Martin NG. Genetic and environmental contributors to alcohol dependence risk in a natural twin sample: consistency of findings in women and men. Psychological Med. 1997;27:1381–1396. doi: 10.1017/s0033291797005643. [DOI] [PubMed] [Google Scholar]
- 15.Woodside DB, Garfinkel PE. Age of onset in eating disorders. Int J Eat Disord. 1992;12:31–36. [Google Scholar]
- 16.Rice JP, Rochberg N, Endicott J, Lavori PW, Miller C. Stability of psychiatric diagnoses. An application of the affective disorders. Arch Gen Psychiatry. 1992;49:824–830. doi: 10.1001/archpsyc.1992.01820100068012. [DOI] [PubMed] [Google Scholar]
- 17.Baker LA, Treloar SA, Reynolds CA, Heath AC, Martin NG. Genetics of educational attainment in Australian twins: Sex differences and secular changes. Behavior Gen. 1996;26:89–102. doi: 10.1007/BF02359887. [DOI] [PubMed] [Google Scholar]
- 18.Eaves LJ, Eysenck HJ, Martin NG, Jardine R, Heath AC, Feingold L, Young PA, Kendler KS. Genes, Culture and Personality: An Emprical Approach. Oxford University Press; London: 1989. [Google Scholar]
- 19.Duffy D. Asthma and allergic diseases in Australian twins and their families. The University of Queensland; Brisbane, Australia: 1994. Unpublished doctoral dissertation. [Google Scholar]
- 20.Bucholz K, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrook VM, Nurnberger JI, Riech T, Schmit F, Shuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Studies Alcohol. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- 21.World Health Organisation. The Composite International Diagnostic Interview 1-1 –Interviewer Manual. American Psychiatric Press Inc; Washington DC: 1993. [Google Scholar]
- 22.Neale MC. Mx: Statistical Modeling. 2. Richmond, VA: Medical College of Virginia. Department of Psychiatry; 1997. [Google Scholar]
- 23.Joreskög KG, Sörbom D. Prelis 2: User’s Reference Guide. Chicago, Il: Scientific Software International; 1996. [Google Scholar]
- 24.Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. [Google Scholar]
- 25.Bulik CM, Sullivan PF, Wade TD, Kendler KS. Twin studies of eating disorders: A review. Int J Eat Disord. 2000;27:1–20. doi: 10.1002/(sici)1098-108x(200001)27:1<1::aid-eat1>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 26.Wade TD, Bulik CM, Neale MC, Kendler KS. Anorexia nervosa and major depression: An examination of shared genetic and environmental risk factors. Am J Psychiatry. 2000;157:469–471. doi: 10.1176/appi.ajp.157.3.469. [DOI] [PubMed] [Google Scholar]
- 27.Bulik CM, Sullivam PF, Tozzi F, Furberg H, Lichtenstein P, Pedersen NL. Prevalence, heritability, and prospective risk factors for anorexia nervosa. Arch Gen Psychiatry. 2006;63:305–312. doi: 10.1001/archpsyc.63.3.305. [DOI] [PubMed] [Google Scholar]
- 28.Klump KL, Miller KB, Keel PK, McGue M, Iacono WG. Genetic and environmental influences on anorexia nervosa syndromes in a population-based twin sample. Psychological Med. 2001;31:737–740. doi: 10.1017/s0033291701003725. [DOI] [PubMed] [Google Scholar]
- 29.Bulik CM, Sullivan PF, Kendler KS. Heritability of binge-eating and broadly defined bulimia nervosa. Biol Psychiatry. 1998;44:1210–1218. doi: 10.1016/s0006-3223(98)00280-7. [DOI] [PubMed] [Google Scholar]
- 30.Fairburn CG, Cooper Z. The Eating Disorder Examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature, assessment and treatment. 12. New York: Guilford Press; 1993. pp. 317–331. [Google Scholar]
- 31.Wade TD, Tiggemann M, Martin NG, Heath AC. A comparison of the Eating Disorder Examination and a structured psychiatric schedule. Australian NZJ Psychiatry. 1997;31:852–857. doi: 10.3109/00048679709065511. [DOI] [PubMed] [Google Scholar]
- 32.Field AE, Colditz GA, Herzog DB, Heatherton TF. Disordered eating: Can women accurately recall their binging and purging behaviours 10 years later? Obesity Res. 1996;4:153–159. doi: 10.1002/j.1550-8528.1996.tb00527.x. [DOI] [PubMed] [Google Scholar]
- 33.Kendler KS. Twins studies of psychiatric illness. Arch Gen Psychiatry. 1993;50:905–915. doi: 10.1001/archpsyc.1993.01820230075007. [DOI] [PubMed] [Google Scholar]