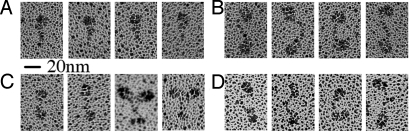
Fig. 3.
Rotary shadowing EM of myosin VI constructs. Representative images for each myosin VI complex are shown. (A) Monomeric myosin VI appears as a single globular motor domain and a narrow lever arm. With the addition of adaptor proteins (either optineurin or truncated Dab-2) dimers are found. (B) Dimeric myosin VI/optineurin molecules are mainly found in a linear configuration with the two globular motor domains separated by ≈180° and the lever arms connected at the tail tips. The distances between heads along the dimer are slightly variable, probably due to different positions of the molecule on the mica surface. The optineurin molecule is not resolved. (C) Myosin VI dimers formed by addition of truncated Dab-2 are in two different configurations: V-shaped and linear with higher prevalence of the latter. The two differ in the position of the heads, which are located at a smaller angle in the V configuration. Note that occasionally myosin VI dimerized in the presence of tDab-2 shows two globular structures at the tail end (C, column 3), likely corresponding to tDab-2 attached to the cargo-binding region. (D) As a comparison, the myosin VI zippered dimer constructs also show two distinct globular heads connected by a common tail region and present either a linear or a V-shaped configuration (5).