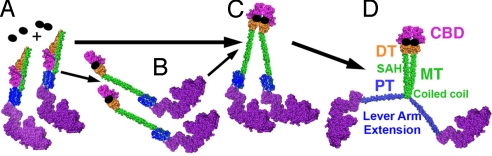
Fig. 4.
Model for cargo-mediated dimerization of myosin VI. Our working model for myosin VI function in a cell is that the full-length myosin VI molecule primarily exists as a monomer folded in such a manner as to form intramolecular interactions involving the cargo-binding domain (magenta) that block potential dimerization sites, as shown in A. This is consistent with small-angle X-ray scattering data obtained by Spink et al. (18). (B) Binding to monomeric cargo adaptors (represented by single black ovals) leads to an unfolding of the monomers, exposing potential dimerization sites. (C) The unfolded monomers can then be held in close proximity, either via tethering by the adaptor protein (25) and/or via as yet unidentified cargo-binding domain interactions. Alternatively, binding to a dimeric cargo adaptor protein (represented by black oval doublet) leads to simultaneous unfolding and close opposition of the cargo-binding domains. (D) This distal tethering of two cargo-binding domains allows internal dimerization (likely coiled coil) to occur at the proximal end of the medial tail (MT), and may include part of the last helix of the three-helix bundle (not depicted). This internal dimerization causes the three-helix bundle, formerly known as the proximal tail (PT), to unfold, forming an extension of the myosin VI lever arm (6).