Abstract
Study Objectives:
To evaluate endothelium-dependent flow-mediated dilation (FMD) and endothelium–independent nitroglycerin (NTG)-induced dilation of the brachial artery with Doppler ultrasound in patients with obstructive sleep apnea (OSA) and impact of six months of continuous positive airway pressure (CPAP) treatment.
Design:
A prospective, controlled, observational study.
Setting:
Single-site, clinic-based.
Patients:
Twenty-nine normotensive men with OSA (apnea-hypopnea index [AHI], mean ± SD, 60.4 ± 22.1-h), and 17 men without OSA (AHI 2.5 ± 0.6-h).
Interventions:
Six months of CPAP therapy in OSA patients.
Measurements and Results:
FMD was lower in patients with OSA compared with in controls (7.19 ± 1.78 % vs 10.93 ± 2.59 %; P < 0.001) while NTG-induced vasodilation was similar in both groups (13.75 ± 1.01 % vs 14.25 ± 1.83 %; n.s.). An inverse relationship was found between FMD and AHI adjusted for age and body mass index (BMI) (β = − 0.05, P < 0.001). Following 6 months of CPAP treatment in the OSA group, FMD was increased from 7.38 ± 2.06 % to 10.45 ± 1.68; P = 0.001) in 20 patients compliant with the device whereas the corresponding values did not change in the non-user group (7.08 ± 1.50% vs 7.26 ± 1.01%). No significant changes were observed regarding the NTG–induced vasodilation after CPAP compared with the baseline values.
Conclusion:
Our results confirm the previous reports suggesting impaired endothelium-dependent FMD in OSA, and additionally document the sustained improvement in endothelial function after 6 months of CPAP treatment in complaint patients.
Citation:
Bayram NA; Ciftci B; Keles T; Durmaz T; Turhan S; Bozkurt E; Peker Y. Endothelial function in normotensive men with obstructive sleep apnea before and 6 months after CPAP treatment. SLEEP 2009;32(10):1257-1263.
Keywords: Obstructive sleep apnea syndrome, flow-mediated dilation, endothelial function, continuous positive airway pressure treatment
OBSTRUCTIVE SLEEP APNEA (OSA) IS A COMMON CONDITION AFFECTING APPROXIMATELY 4% OF THE ADULT MALE POPULATION, CHARACTERIZED BY repetitive intermittent collapse of the upper airway during sleep.1 There is growing research evidence suggesting an increased cardiovascular morbidity and mortality in OSA patients.2,3 Although extensive experimental and clinical data have demonstrated profound immediate autonomic and hemodynamic changes during apneic events, the long-term vascular consequences of OSA are still incompletely characterized. Vascular endothelial dysfunction and structural vascular changes have been implicated as early mechanisms in the pathophysiology of hypertension and other forms of vascular disease.4 Previous work has also associated an attenuated flow-mediated dilation (FMD) with OSA.5–9 In recent controlled trials, elimination of apneic events by continuous positive airway pressure (CPAP) in patients with OSA has been shown to reduce blood pressure.10,11 Similarly, CPAP treatment has been shown to affect other markers of early atherogenic processes.12–14 Regarding the impact of CPAP on FMD in patients with OSA, there is 1 report after short-term (4-week) treatment.9 Moreover, in a smaller group of patients OSA, vasodilator response to acetylcholine was improved after 3 months of treatment.15 To our knowledge, there are no data on whether the improvement in endothelial function is sustainable over the long term. In the current prospective observational study, we addressed the impact of 6 months of CPAP treatment on endothelial function in men with OSA.
METHODS
Study Population
All participants were recruited among the subjects who were thought to potentially have OSA and who were seen at the Sleep Disorders Center of the Ataturk Chest Diseases and Chest Surgery Research and Training Hospital (Ankara, Turkey) between January 1, 2005 and July 31, 2006. The subjects were eligible if they were willing to participate in the study and if they were known not to have hypertension or other cardiovascular disease, diabetes mellitus, dyslipidemia, alcoholism, neuromuscular disease, renal failure, chronic obstructive pulmonary disease, or malignancy; were not previously diagnosed with or treated for OSA; and did not use any medication. Current smokers and exsmokers (who had stopped smoking at least 6 months prior to the admission to the sleep clinic) were defined as smokers, whereas subjects who never smoked were defined as nonsmokers. Only men were included. Subjects with a total sleep time shorter than 240 minutes and those with signs of central sleep apnea on polysomnography recordings were not included.
The Ethics Committee of the local hospital approved the study protocol, and informed consent was obtained from each participant prior to the study entry.
Overnight Sleep Studies
All participants underwent a full-night polysomnogram, using the Compumedics Voyager Digital Imaging E-series system (Compumedics®, Melbourne, Victoria, Australia). The subjects were advised to not sleep during the day, to not consume caffeine-containing beverages, and to not take medication that might affect the sleeping pattern. The polysomnography recordings included 2-channel electroencephalography, 2-channel electrooculography, 1-channel submental electromyography, oxygen saturation via an oximeter probe, respiratory movements via chest and abdominal belts, nasal pressure via pressure sensor, electrocardiography, and leg movements via tibial surface electrodes. Sleep stages as well as respiratory parameters were scored according to the standard criteria of American Academy of Sleep Medicine.16 Apnea was defined as a complete cessation of airflow for at least 10 seconds. Hypopnea was defined as a reduction in airflow of at least 50% accompanied by at least a 3% desaturation in the preceeding 30 seconds and a reduction in chest wall movement and/or arousal. The apnea-hypopnea index (AHI) referred to the average number of apneas and hypopneas per hour of sleep. Subjects with an AHI of less than 5 per hour were defined as not having OSA and were included in the study as control subjects. Patients with an AHI of 15 or greater per hour were defined as having OSA and were offered CPAP treatment. Subjects with an AHI of at least 5 but less than 15 per hour were not included in the study because CPAP treatment was not indicated as first-line treatment in otherwise healthy individuals, according to our clinic routines.
Polysomnography recordings were redone during the first night on CPAP treatment by autotitrating CPAP devices (S8®, ResMed Ltd, Sydney, Australia or Respironics REMstar®, Plus M Series w/C-flex™ Respironics, Inc., Murrysville, PA, USA) according to clinic standards with a nasal or full-face mask and humidifier by trained staff. Subjects demonstrating central apneas during the titration night were not included in the study because of the debated issue regarding the complex sleep apnea syndromes and risk for low compliance with CPAP.17 All study patients assigned to CPAP were instructed to use CPAP at home every night for at least 4 hours, contacted by telephone after 1 week, and given a check-up in the clinic after 1 month, 3 months, and 6 months. Patients were considered to be CPAP compliant if they used the device (documented by the time-counter of the device) at least 4 hours per night and 5 days per week.18 Six months after the study start, a new overnight polysomnogram was conducted on the CPAP users to ensure the treatment efficacy before the measurement of endothelial function. No new polysomnogram was done in the nonuser group.
Assessment of Endothelial Function
Patients were asked to abstain from alcohol, tobacco, and caffeine on the day of the measurements. A high-resolution 12.0-MHz linear array transducer ultrasound (Vingmed System 7; Vivid 7 Pro; Horten, Norway) was used to measure brachial FMD. Ultrasound examinations were assessed in supine position at stable room temperature between 21°C and 25°C in the morning (between 0800 and 0900) after at least a 10-hour fast. All participants abstained from vitamin C intake, fatty meals, smoking, and consumption of caffeine-containing drinks for at least 12 hours before testing. B-mode longitudinal sections were obtained between 2 and 10 mm above the antecubital fossa.19 After baseline measurements of brachial artery diameter and flow velocity, a blood pressure cuff was inflated proximal to the section of the brachial artery to an occlusive pressure (50 mm Hg higher than systolic blood pressure or 250 mm Hg) for 5 minutes to induce reactive hyperemia. The cuff was then rapidly deflated. Flow velocity was recorded for 15 seconds, and brachial artery diameter was measured for 60 seconds after cuff deflation. FMD was expressed as the percentage change in brachial artery diameter from baseline following reactive hyperemia (FMD % = [Brachial artery diameter {reactive hyperemia} - brachial artery diameter {baseline}] / brachial artery diameter [baseline] x 100). After recovery within 15 minutes, baseline brachial artery diameters were measured again, and sublingual nitroglycerine was administered. Brachial artery diameter and flow velocity were obtained after 3 minutes. Nitroglycerine-mediated vasodilation was defined as the percentage change in brachial artery diameter from baseline following nitroglycerine-induced vasodilation (nitroglycerine-induced dilation % = [brachial artery diameter {nitroglycerine-induced vasodilation} - brachial artery diameter {baseline}] / brachial artery diameter [baseline] x 100). All measurements of the brachial artery internal diameter were assessed at the end of the diastolic phase (timed by the QRS complex) and were calculated as the average of the measurements obtained during 3 consecutive cardiac cycles.
All measurements were assessed by 2 independent cardiologists blinded to the sleep recordings of the subjects, and the average values were taken. Endothelial function measurements were reassessed after 6 months in the OSA group by the same cardiologists blinded to the previous FMD results as well as to the CPAP-compliance data.
Statistical Analysis
Continuous variables were expressed as means ± standard deviation (SD), and categorical variables as numbers and proportions (%). Assumption of normal distribution seemed not to be violated when investigated visually by histogram and by Kolmogorov-Smirnov normality test. For comparison of the groups, an independent student t test was applied for the continuous variables, and the χ2 or Fisher exact test for the categorical variables. In the OSA group, paired-samples t test was used for comparison of the FMD values before and after CPAP treatment. Relationships between variables were assessed with the Pearson or Spearman correlation test. Multiple linear regression analyses, with the FMD as the dependent variable, were performed to evaluate the independent contributions of AHI, age, and body mass index (BMI). All statistical tests were 2 sided, and a P value of less than 0.05 was considered significant. The analyses were assessed with the Statistical Package for Social Sciences version 15.0 for Windows® system (SPSS® Inc., Chicago, IL).
RESULTS
Out of 63 consecutive men undergoing polysomnography, 9 demonstrated an AHI between 5 and 15per hour. Two individuals among 19 without OSA (control group) refused further investigations and were excluded from further analysis, as were 4 of the 35 patients with OSA who refused CPAP. Two patients with OSA who demonstrated central apneas during the CPAP titration night were also excluded. Remaining 46 men (29 OSA, 17 non-OSA) were included in the final study population.
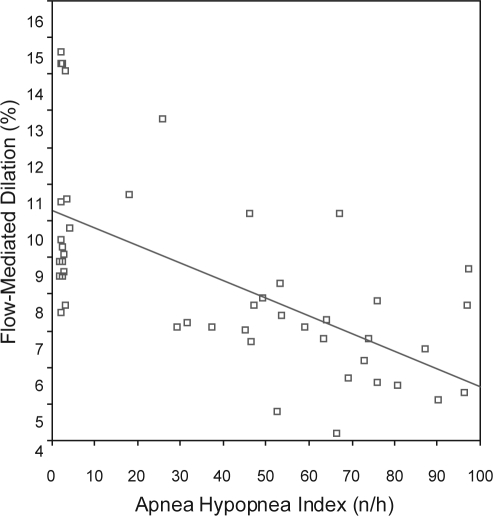
When comparing patients with OSA and control subjects, there were no significant differences regarding age, BMI, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP) total cholesterol, low-density-lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, triglycerides, fasting glucose levels, and the proportion of smokers (Table 1). The control group—with an AHI less than 5 per hour—were mainly snorers without excessive daytime sleepiness or other significant sleep complaints. As shown in Table 2, the brachial artery diameter at baseline was similar in patients with OSA and control subjects, and there was an increase in the diameter in both groups in response to reactive hyperemia. However, the FMD was significantly lower in patients with OSA than was the FMD in control subjects (7.19 ± 1.78% vs 10.93 ± 2.59%; P < 0.001) There was no significant difference between the groups regarding the nitroglycerine-mediated vasodilation. Pearson correlation analysis revealed an inverse relationship between FMD and age (r = −0.35, P = 0.016) and BMI (r = −0.34, P = 0.02). No significant relationship was found between FMD and heart rate, SBP, DBP, lipids, or fasting glucose levels in this population. As illustrated in Figure 1, there was a negative partial correlation between FMD and AHI (r = −0.63, P < 0.001; β = −0.048). After including age and BMI in the model, the association between AHI and FMD was still statistically significant (β = −0.046, P < 0.001). The total variation explained by the model was around 50% (R2 = 0.51).
Table 1.
Clinical Characteristics of Patients with OSA and Control Subjects at Baseline
| OSA patients (n = 29) | control subjects (n = 17) | P Value | |
|---|---|---|---|
| Age, y | 43.7 ± 8.2 | 43.4 ± 8.9 | NS |
| BMI, kg/m2 | 30.2 ± 5.2 | 27.8 ± 4.0 | NS |
| Blood pressure, mm Hg | |||
| Systolic | 127.4 ± 11.4 | 127.7 ± 7.3 | NS |
| Diastolic | 79.3 ± 7.0 | 79.9 ± 7.3 | NS |
| Heart rate, beats/min | 73.9 ± 9.4 | 70.2 ± 8.6 | NS |
| Cholesterol, mg/dL | |||
| Total | 167.2 ± 11.7 | 168.8 ± 15.2 | NS |
| LDL | 93.7 ± 14.0 | 89.2 ± 13.5 | NS |
| HDL | 39.0 ± 3.7 | 39.7 ± 3.3 | NS |
| Triglycerides, mg/dL | 129.5 ± 19.1 | 128.2 ± 18.8 | NS |
| Fasting serum glucose, mg/dL | 101.7 ± 12.4 | 101.5 ± 13.7 | NS |
| Smoking status, n (%) | |||
| Current | 8 (27.6) | 7 (41.2) | NS |
| Former | 6 (20.7) | 3 (17.7) | NS |
| AHI, n/h | 60.4 ± 22.1 | 2.5 ± 0.6 |
Data are presented as mean ± SD unless otherwise indicated. OSA refers to obstructive sleep apnea; BMI, body mass index; LDL, low-density lipoprotein; HDL, high-density lipoprotein; AHI: apnea hypopnea index; NS: not significant
Table 2.
Endothelium-Dependent FMD and Endothelium-Independent NMD in Patients with OSA and Control Subjects at Baseline
| Patients with OSA | Control subjects | P Value | |
|---|---|---|---|
| FMD | |||
| Brachial artery diameter, mm | |||
| Baseline | 4.18 ± 0.24 | 4.07 ± 0.32 | 0.210 |
| Reactive hyperemia | 4.48 ± 0.27 | 4.51 ± 0.34 | 0.712 |
| FMD, % | 7.19 ± 1.78 | 10.93 ± 2.59 | < 0.001 |
| NMD | |||
| Brachial artery diameter, mm | |||
| Baseline | 4.18 ± 0.45 | 4.23 ± 0.26 | 0.530 |
| Reactive hyperemia | 4.75 ± 0.27 | 4.74 ± 0.31 | 0.951 |
| NMD, % | 13.75 ± 1.01 | 14.25 ± 1.83 | 0.236 |
FMD refers to flow-mediated dilation; NMD; nitroglycerin-mediated dilation; OSA, obstructive sleep apnea.
Figure 1.
Correlation between the apnea hypopnea index and endothelium-dependent flow-mediated dilation in normotensive men (r = −0.63, P < 0.001)
In the whole OSA group, there was a significant increase in FMD after 6 months of CPAP treatment (7.29 ± 1.88% vs 9.46 ± 2.11%; P = 0.002). Among 29 patients with OSA who were receiving CPAP, 20 were found to be CPAP users (5.9 ± 0.1 hour/day) and 9 were nonusers (3 returned the device within 2 weeks, 6 within 1 month). Baseline characteristics of the CPAP users and nonusers regarding age (44.3 vs 42.6 years), BMI (30.5 vs 31.0 kg/m2), AHI (59.7 vs 62.1/h), FMD (7.38 vs 7.08%), as well as the average CPAP pressure (9.5 vs 9.0 mbar), did not differ significantly. At the 6-month follow-up, there were no significant changes in BMI in either group. Two current smokers at baseline in the CPAP-user group and 1 in the nonuser group were categorized as exsmokers at follow-up because they had quit smoking before the study start (see definitions for exsmokers in Methods above).
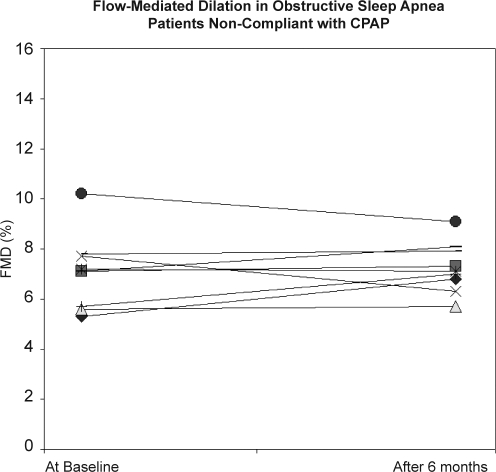
The patients with OSA in the CPAP-user group were effectively treated with regard to obstructive events, as verified on the overnight polysomnogram at 6-month follow-up (Table 3). None of the CPAP users demonstrated central apneas on treatment. As shown in Table 4 and illustrated in Figure 2 a.b, the FMD was increased from 7.38 ± 2.06% to 10.45 ± 1.68% (P = 0.001), whereas the corresponding values were not significantly improved (7.08 ± 1.50% vs 7.26 ± 1.01%) in the nonuser group. Moreover, there was a positive correlation between CPAP hours and FMD values following CPAP treatment (r = 0.76, P < 0.001) in the CPAP-user group. No significant changes were observed regarding nitroglycerine-induced vasodilation after 6 months of CPAP treatment, compared with the baseline values (Table 4).
Table 3.
Changes in Polysomnographic Respiratory Parameters of the Patients with Obstructive Sleep Apnea Before and After 6 Months on CPAP
| Before CPAP | On CPAP | P Value | |
|---|---|---|---|
| AHI, n/h | 62.4 ± 23.5 | 3.3 ± 1.5 | < 0.001 |
| SaO2, % | |||
| Mean | 89.6 ± 4.4 | 92.8 ± 2.1 | 0.040 |
| Minimum | 72.8 ± 11.7 | 87.4 ± 4.6 | 0.003 |
| Time spent < 90% saturation, min | 28.2 ± 26.2 | 1.5 ± 3.4 | < 0.001 |
OSA refers to obstructive sleep apnea; CPAP, continuous positive airway pressure; AHI, apnea-hypopnea index; SaO2, arterial oxygen saturation.
Table 4.
Changes in FMD and NMD in Compliant Patients with OSA Before and 6 Months Following CPAP Treatment
| Before CPAP | After CPAP | P Value | |
|---|---|---|---|
| FMD | |||
| Brachial artery diameter, mm | |||
| Baseline | 4.19 ± 0.25 | 4.21 ± 0.25 | 0.450 |
| Reactive hyperemia | 4.50 ± 0.28 | 4.64 ± 0.26 | < 0.001 |
| FMD, % | 7.38 ± 2.06 | 10.45 ± 1.68 | < 0.001 |
| NMD | |||
| Brachial artery diameter, mm | |||
| Baseline | 4.19 ± 0.42 | 4.21 ± 0.36 | 0.480 |
| Reactive hyperemia | 4.77 ± 0.27 | 4.79 ± 0.28 | 0.216 |
| NMD, % | 13.97 ± 0.87 | 13.85 ± 1.11 | 0.649 |
FMD refers to flow-mediated dilation; NMD; nitroglycerin-mediated dilation; OSA, obstructive sleep apnea; CPAP, continuous positive airway pressure.
Figure 2a.
Baseline and 6-month individual values of flow-mediated dilation (FMD) in patients with obstructive sleep who were compliant with continuous positive airway pressure therapy. Symbols indicate different individuals.
Figure 2b.
Baseline and 6-month individual values of flow-mediated dilation (FMD) in patients with obstructive sleep apnea who were not compliant with continuous positive airway pressure therapy. Symbols indicate different individuals.
DISCUSSION
The current study confirms the previous reports suggesting an impaired endothelium-dependent FMD in patients with OSA and additionally demonstrates that the reversal of this dysfunction is consistent after 6 months of CPAP therapy if the patients are compliant with the treatment.
Endothelial dysfunction is one of the early markers of atherosclerosis and is associated with increased cardiovascular events.20 It is shown that endothelial dysfunction develops in patients with cardiovascular risk factors such as hypertension, diabetes mellitus, hyperlipidemia, obesity, and smoking before the atherosclerosis findings develop and that endothelial dysfunction can be restored with treating these risk factors.21–23 FMD in the brachial artery is the most commonly used noninvasive method for assessing endothelial function. Using similar techniques, hyperemic FMD in the brachial artery has been demonstrated to reflect endothelial function,19 mainly mediated by endothelial nitric oxide (NO).24 In a metaregression analysis of 211 selected articles (399 populations) reporting on FMD and cardiovascular risk factors, low FMD values were shown to be associated with a 10-year risk of coronary artery disease in low-risk populations, based on the Framingham risk score.25
It has also been shown that atherosclerosis is common in patients with OSA with alterations in mediators such as NO, cell adhesion molecules, and fibrinogen, which are markers of endothelial dysfunction.26–28 It has also been suggested that recurrent hypoxia-reoxygenation during apneas and hypopneas and increased sympathetic activity cause stress in endothelium and lead to the release of NO.29,30 Sympathetic stimulation, at a clinically relevant range, has been demonstrated to impair the FMD response by an α-adrenergic mechanism.31
In previous studies addressing the vascular endothelial function in OSA, the results have not been fully conclusive. Blunted vasodilation in response to infusion of acetylcholine, a vasodilator that stimulates endothelial release of NO, has been demonstrated in forearm resistance vessels using occlusion plethysmography,32 but these results were not confirmed in a later study.33 Using ultrasound Doppler, impairment of FMD in the brachial artery has been reported to correlate with minimum oxygen saturation in subjects with OSA.34 It should also be added that the ultrasound Doppler technique has been shown to be accurate and reproducible.35 Nieto et al.5 showed, also using this technique, that endothelial function is impaired in patients with OSA and that FMD levels decrease as the severity of the disease progresses, particularly in elderly patients (in this study, > 68 yrs). In our study, a significant inverse relationship was seen between FMD and the severity of OSA in terms of AHI (r = −0.63, P < 0.001). In the study of Tanrverdi et al.6 comparing aortic stiffness, carotid intima media thickness, and FMD levels, the FMD was shown to be lower, compared with the levels in control subjects. In the study of Oflaz and coworkers, the FMD was also impaired in normotensive patients with OSA, compared with healthy control subjects.7 Moreover, a prominent diurnal deterioration with even lower values in the morning was reported in that study.7 It should also be added that the impaired FMD was not confined to only the patients with OSA and excessive daytime sleepiness. Kohler and coworkers demonstrated a significantly lower FMD in subjects with minimally symptomatic OSA, compared with the FMD values in a well-matched control group.8
The first-line treatment for patients with OSA is CPAP therapy.36 Intensive research has shown that sleep pattern is restored; snoring disappears and daytime sleepiness disappear; AHI and arousal decrease; oxygen saturation is normalized; excessive thrombocyte activation, plasminogen, and blood viscosity increases are prevented; NO levels decrease; hypertension is restored; and pulmonary artery pressure decreases with CPAP treatment.37 Less is known regarding the impact of CPAP on endothelial function. Ip and coworkers demonstrated that the impaired FMD was significantly improved after 4 weeks of CPAP treatment in 28 men with moderate to severe OSA.9 Interestingly, 8 subjects who used CPAP for more than 3 months were reassessed after the treatment was withdrawn for 1 week, and FMD values became lower than during treatment and were similar to baseline values. The authors speculated that endothelial dysfunction is related to relatively acute pathophysiologic changes in otherwise healthy normotensive men.9
Additionally, the endothelium-dependent dilation to acetylcholine in forearm blood flow has been shown to be significantly increased after 3 months of CPAP treatment in a smal group of subjects with OSA.15 Our results confirm the beneficial impact of CPAP in this context extended to a longer time period, such as 6 months, if the patients are compliant with the treatment. Moreover, compared with the findings in the study of Ip and coworkers,9 the improvement in FMD after 6 months of treatment in the current paper may support the hypothesis that the benefit of the CPAP treatment is a sustainable improvement rather than a gradual deterioration after the initial improvement. It should also be added that the improvement in FMD was positively correlated with the objective CPAP use.
One limitation of our study refers to the lack of information regarding the physical activity before and after CPAP treatment in patients with OSA because increased physical activity has been shown to play an important role in endothelial function.38 However, there were no significant changes in body weight in either group during the observation period, which might indirectly reflect that there were no significant changes in the physical activity. The change in the smoking habits in 3 individuals in the whole cohort probably had no significant impact on the group level with regard to FMD values at follow-up.
Because subjects were excluded if they were known to have hypertension, cardiovascular disease, diabetes mellitus, dyslipidemia, alcoholism, neuromuscular disease, renal failure, chronic obstructive pulmonary disease, or malignancy—and no subjects were taking medication—one may argue that these are very strict selection criteria. Although the selection criteria certainly help to eliminate confounding from these associated conditions, they also introduce an inherent bias in selection and may deviate significantly from the clinically relevant common situation in which these factors cluster and coexist with obesity and OSA. However, because obese subjects as well as smokers were not excluded, the current cohort represents an otherwise-healthy sleep-clinic population. On the other hand, it should be emphasized that the subjects in the current study were men with moderate to severe OSA, and the findings may not extrapolate to women and to those with mild OSA. It should also be kept in mind that endothelial dysfunction starts physiologically a decade earlier in men than in women.39 Moreover, the endothelial function varies in women during the menstrual cycle and after menopause,40 which makes it difficult to control as a confounding factor when addressing the impact of OSA as well the impact of treatment on the vascular endothelium. We have therefore not included women in the current study. However, sex differences in the cardiovascular complications of OSA is another challenging issue, which should be considered in future studies.
In conclusion, awaiting the results of randomized controlled intervention trials for a causal relationship between OSA and endothelial dysfunction, we confirm that the endothelium-dependent FMD is impaired in normotensive men with moderate to severe OSA and additionally suggest that the reversal of this dysfunction is consistent after 6 months of CPAP treatment in compliant patients.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank statistician Salmir Nasic at the Research Center of the Skaraborg Hospital for help in analyzing the results.
REFERENCES
- 1.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–1239. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 2.Peker Y, Hedner J, Norum J, Kraiczi J, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166:159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 3.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 4.Furchgott RF. Role of endothelium in responses of vascular smooth muscle. Circ Res. 1983;53:557–573. doi: 10.1161/01.res.53.5.557. [DOI] [PubMed] [Google Scholar]
- 5.Nieto FJ, Herrington DM, Redline S, Benjamin EJ, Robbins JA. Sleep apnea and markers of vascular endothelial function in a large community sample of older adults. Am J Respir Crit Care Med. 2004;169:354–360. doi: 10.1164/rccm.200306-756OC. [DOI] [PubMed] [Google Scholar]
- 6.Tanrverdi H, Evrengul H, Kara CO, et al. Aortic stiffness, flow-mediated dilatation and carotid intima-media thickness in obstructive sleep apnea: non-invasive indicators of atherosclerosis. Respiration. 2006;73:741–750. doi: 10.1159/000093531. [DOI] [PubMed] [Google Scholar]
- 7.Oflaz H, Cuhadaroglu C, Pamukcu B, et al. Endothelial function in patients with obstructive sleep apnea syndrome but without hypertension. Respiration. 2006;73:751–756. doi: 10.1159/000094183. [DOI] [PubMed] [Google Scholar]
- 8.Kohler M, Craig S, Nicoll D, Leeson P, Davies RJ, Stradling JR. Endothelial function and arterial stiffness in minimally symptomatic obstructive sleep apnea. Am J Respir Crit Care Med. 2008;178:984–988. doi: 10.1164/rccm.200805-717OC. [DOI] [PubMed] [Google Scholar]
- 9.Ip MSM, Tse HF, Lam B, Tsang KWT, Lam WK. Endothelial function in obstructive sleep apnea and response to treatment. Am J Respir Crit Care Med. 2004;169:348–353. doi: 10.1164/rccm.200306-767OC. [DOI] [PubMed] [Google Scholar]
- 10.Pepperell JC, Ramdassingh-Dow S, Crosthwaite N, et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomized parallel trial. Lancet. 2002;359:204–210. doi: 10.1016/S0140-6736(02)07445-7. [DOI] [PubMed] [Google Scholar]
- 11.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 12.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 13.Imadojemu VA, Gleeson K, Quraishi SA, Kunselman AR, Sinoway LI, Leuenberger UA. Impaired vasodilator responses in obstructive sleep apnea are improved with continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2002;165:950–953. doi: 10.1164/ajrccm.165.7.2102003. [DOI] [PubMed] [Google Scholar]
- 14.Ciftci TU, Kokturk O, Bukan N, Bilgihan A. The relationship between serum cytokine levels with obesity and obstructive sleep apnea syndrome. Cytokine. 2004;28:87–91. doi: 10.1016/j.cyto.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Lattimore JL, Wilcox I, Skilton M, Langenfeld M, Celermayer D. Treatment of obstructive sleep apnoea leads to improved microvascular endothelial function in the systemic circulation. Thorax. 2006;61:491–495. doi: 10.1136/thx.2004.039164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.American Academy of Sleep Medicine Task Force Report. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 17.Morgenthaler TI, Kagramanov V, Hanak V, Decker PA. Complex sleep apnea syndrome: is it a unique clinical syndrome? Sleep. 2006;29:1203–1209. doi: 10.1093/sleep/29.9.1203. [DOI] [PubMed] [Google Scholar]
- 18.Richard W, Venker J, den Herder C, et al. Acceptance and long-term compliance of nCPAP in obstructive sleep apnea. Eur Arch Otorhinolaryngol. 2007;264:1081–1086. doi: 10.1007/s00405-007-0311-3. [DOI] [PubMed] [Google Scholar]
- 19.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 20.Vita JA, Keaney JF., Jr. Endothelial function: a barometer for cardiovascular risk? Circulation. 2002;106:640–642. doi: 10.1161/01.cir.0000028581.07992.56. [DOI] [PubMed] [Google Scholar]
- 21.Tounian P, Aggoun Y, Dubern B, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 22.Clarkson P, Celermajer DS, Donald AE, et al. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. J Am Coll Cardiol. 1996;28:573–579. doi: 10.1016/0735-1097(96)82380-1. [DOI] [PubMed] [Google Scholar]
- 23.Gokce N, Holbrook M, Duffy SJ, et al. Effects of race and hypertension on flow-mediated and nitroglycerin- mediated dilation of the brachial artery. Hypertension. 2001;38:1349–1354. doi: 10.1161/hy1201.096575. [DOI] [PubMed] [Google Scholar]
- 24.Ip MS, Lam B, Chan LY, et al. Circulating nitric oxide is suppressed in obstructive sleep apnea and is reversed by nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2000;162:2166–2171. doi: 10.1164/ajrccm.162.6.2002126. [DOI] [PubMed] [Google Scholar]
- 25.Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow-mediated dilation and cardiovascular risk limited to low-risk populations? J Am Coll Cardiol. 2005;45:1987–1993. doi: 10.1016/j.jacc.2005.02.073. [DOI] [PubMed] [Google Scholar]
- 26.Kato M, Roberts-Thomson P, Phillips BG, et al. Impairment of endothelium-dependent vasodilation of resistance vessels in patients with obstructive sleep apnea. Circulation. 2000;102:2607–2610. doi: 10.1161/01.cir.102.21.2607. [DOI] [PubMed] [Google Scholar]
- 27.Lavie L. Obstructive sleep apnea syndrome: an oxidative stress disorder. Sleep Med Rev. 2003;7:35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 28.Dyugovskaya L, Lavie P, Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165:934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 29.Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanism in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. doi: 10.1172/JCI118235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leuenberger U, Jacob E, Sweer L, Waravdekar N, Zwillich C, Sinoway L. Surges of muscle sympathetic nerve activity during obstructive sleep apnea are linked to hypoxemia. J Appl Physiol. 1995;79:581–588. doi: 10.1152/jappl.1995.79.2.581. [DOI] [PubMed] [Google Scholar]
- 31.Hijmering ML, Stroes ES, Olijhoek J, Hutten BA, Blankestijn PJ, Rabelink TJ. Sympathetic activation markedly reduces endothelium-dependent, flow-mediated vasodilation. J Am Coll Cardiol. 2002;39:683–688. doi: 10.1016/s0735-1097(01)01786-7. [DOI] [PubMed] [Google Scholar]
- 32.Carlson JT, Rangemark C, Hedner JA. Attenuated endothelium-dependent vascular relaxation in patients with sleep apnoea. J Hypertens. 1996;14:577–584. doi: 10.1097/00004872-199605000-00006. [DOI] [PubMed] [Google Scholar]
- 33.Kraiczi H, Hedner J, Peker Y, Carlson J. Increased vasoconstrictor sensitivity in obstructive sleep apnea. J Appl Physiol. 2000;89:493–498. doi: 10.1152/jappl.2000.89.2.493. [DOI] [PubMed] [Google Scholar]
- 34.Kraiczi H, Caidahl K, Samuelsson A, Peker Y, Hedner J. Impairment of vascular endothelial function and left ventricular filling: association with the severity of apnea-induced hypoxemia during sleep. Chest. 2001;119:1085–1091. doi: 10.1378/chest.119.4.1085. [DOI] [PubMed] [Google Scholar]
- 35.Sorensen KE, Celermajer DS, Spiegelhalter DJ, et al. Noninvasive measurement of human endothelium-dependent arterial responses: accuracy and reproducibility. Br Heart J. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Basner RC. Continuous positive airway pressure for obstructive sleep apnea. N Engl J Med. 2007;356:1751–1758. doi: 10.1056/NEJMct066953. [DOI] [PubMed] [Google Scholar]
- 37.Leung RS, Bradley TD. Sleep apnea and cardiovascular disease. Am J Respir Crit Care Med. 2001;164:2147–2165. doi: 10.1164/ajrccm.164.12.2107045. [DOI] [PubMed] [Google Scholar]
- 38.Pahkala K, Heinonen OJ, Lagstrom H, et al. Vascular endothelial function and leisure-time physical activity in adolescents. Circulation. 2008;181:2353–2359. doi: 10.1161/CIRCULATIONAHA.108.791988. [DOI] [PubMed] [Google Scholar]
- 39.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto M, Akishita M, Eto M, et al. Modulation of endothelium-dependent flow-mediated dilatation of the brachial artery by sex and menstrual cycle. Circulation. 1995;92:3431–3435. doi: 10.1161/01.cir.92.12.3431. [DOI] [PubMed] [Google Scholar]