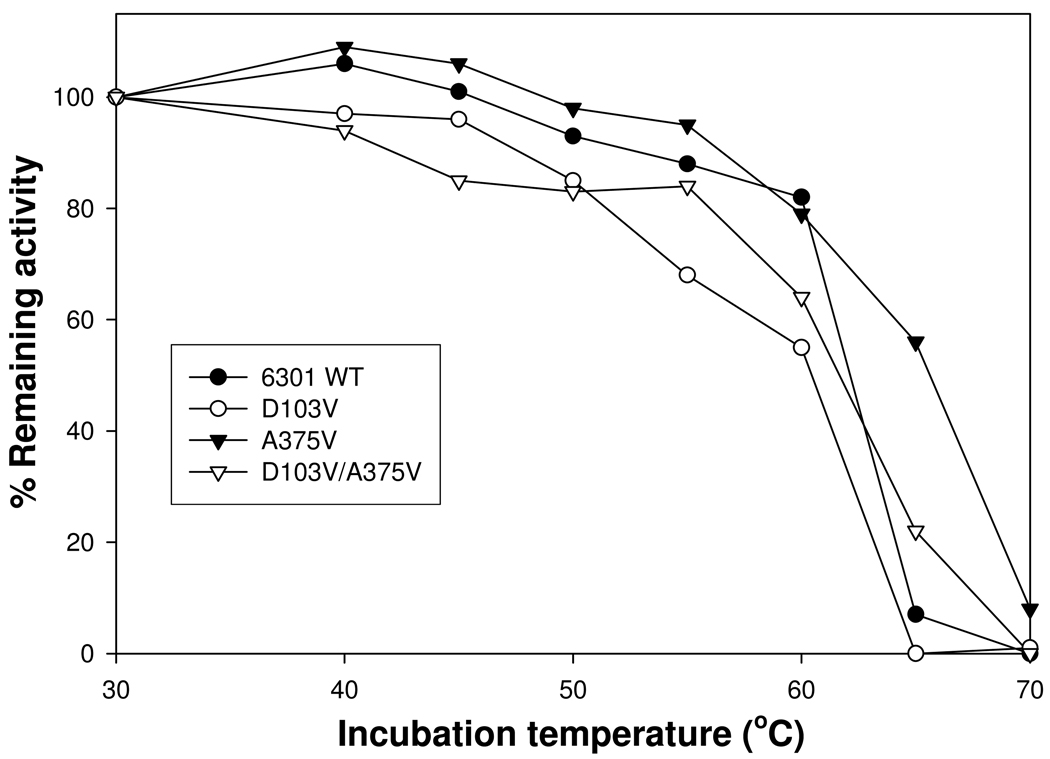
FIGURE 3.
Thermal inactivation of Synechococcus 6301 wild-type and mutant enzymes. Aliquots of each enzyme were incubated at the respective temperatures for 10 min, cooled on ice and assayed for the remaining amount of carboxylase activity. For each enzyme sample, the amounts of activity corresponding to the different incubation temperatures were normalized against the levels of activity calculated from the same enzyme incubated at 30°C. Data is representative of 2 identical experiments that gave similar results. The A375V mutant enzyme appears to retain at least 55% of the original activity after the 65°C incubation, indicating that the mutation likely results in major structural changes that should contribute to the catalytic properties of the enzyme assayed in vitro.