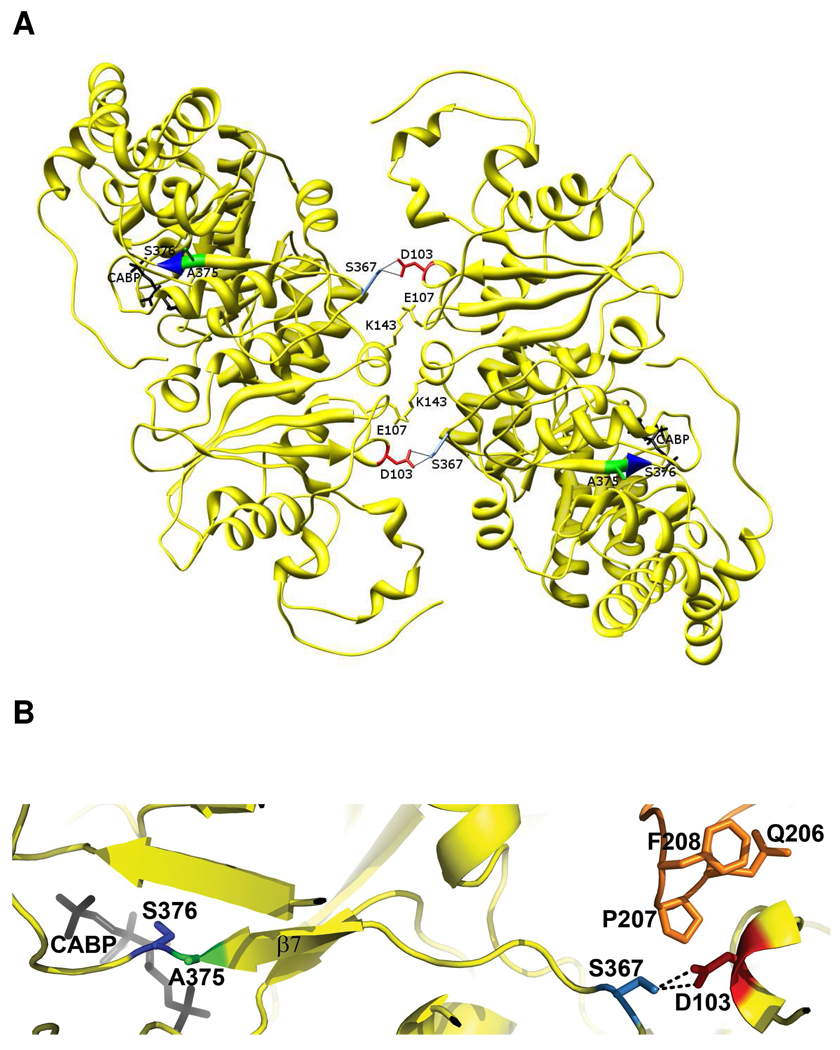
FIGURE 4.
(A) Interaction of Asp-103 (red) from one monomer of a dimer of Synechococcus Rubisco large subunits with Ser-367 (light blue) of a monomer from a different dimer (1RBL) (37). There are two such interactions for each pair of interacting monomers (yellow). (B) Close-up view of the surface interactions of Asp-103 from one subunit with Ser-367 of another subunit and residues Gln-206, Pro-207 and Phe-208 of yet another large subunit (orange). Ser-367 is on the same strand as Ala-375 (green), which contacts active-site Ser-376 (blue). Residues Gln-206 to Phe-208 are only a few residues away from the active-site residues Lys-198, Asp-200 and Asp-201.