Abstract
Granulocyte colony-stimulating factor (G-CSF)-mobilized donor graft tissue used for peripheral blood stem cell transplantation contains a large number of immature myeloid cells that suppress alloreactive donor T cells, resulting in an inhibition of acute graft-versus-host disease (GVHD). However, the molecular mechanism underlying the suppressive function of immature myeloid cells is not fully understood. Here, we investigated whether indoleamine 2,3-dioxygenase (IDO) is related to the suppressive mechanism of G-CSF-induced immature myeloid cells (gMCs). We found that Gr-1+ CD11b+ cells were highly induced in G-CSF-injected donor graft tissue, which is a phenotype of immature myeloid cells, resulting in an inhibition of acute GVHD lethality by suppressing alloreactive donor T-cell expansion. IDO was not detected in primary isolated gMCs; however, this enzyme was markedly induced after treatment with interferon-γ (IFN-γ). This level was significantly higher in IFN-γ-treated gMCs than in bone marrow myeloid cells, which promote alloreactive T-cell responses. We next investigated the functional role of IDO in gMC-mediated inhibition of acute GVHD lethality. We found no changes in gMC-mediated survival or alloreactive donor T-cell suppression when IDO activity was blocked using 1-methyl tryptophan. In addition, there was no difference in gMC-mediated survival rates between recipients transferred with either wild-type gMCs or IDO−/− gMCs. Taken together, our data suggest that gMC-mediated inhibition of lethal acute GVHD is through an IDO-independent mechanism.
Keywords: graft-versus-host disease; granulocyte colony-stimulating factor; immature myeloid cells; indoleamine 2,3-dioxygenase
Introduction
Allogeneic haematopoietic stem cell transplantation (allo-HSCT) is the most effective therapy for several haematological disorders. Recently, peripheral blood stem cells (PBSCs) obtained from granulocyte colony-stimulating factor (G-CSF)-mobilized donors have been used more frequently than bone marrow stem cells as the source of cells in allo-HSCT. The clinical advantages of using PBSCs are more rapid engraftment and immune reconstitution without increasing the incidence of acute graft-versus-host disease (GVHD), despite the fact that a more than 10-fold higher number of mature donor T cells are introduced with the transplant donor graft.1–6 However, the rate of chronic GVHD is higher in PBSC transplantation than it is in bone marrow transplantation.7,8 Therefore, it is possible that an immunoregulatory factor that suppresses acute GVHD is transiently induced with G-CSF.
Not only does G-CSF directly induce T helper type 2 (Th2) polarization of donor T cells but it also indirectly affects this pathway.9,10 It is primarily responsible for the induction of tolerogenic cells, including dendritic cells, monocytes and granulocytes. Monocytes derived from the donor graft of PBSC transplantation have been shown to suppress alloreactive T-cell expansion by increasing interleukin-10 (IL-10) production.11,12 In experimental models, an adoptive transfer of G-CSF-expanded immature myeloid cell populations, promoted transplant tolerance through the generation of antigen-specific regulatory T cells.13 Low-density granulocytes from the peripheral blood of G-CSF-treated donors inhibit acute GVHD.14,15 However, the molecular mechanism mediating these cell types remains unclear.
Indoleamine 2,3-dioxygenase (IDO) is a potent immunoregulatory enzyme that converts the essential amino acid tryptophan into catabolic products collectively known as kynurenines.16–18 The functional activity of IDO in the placenta prevents maternal T-cell immune responses in fetal tissues during pregnancy.19 Therefore, IDO-dependent suppression of T-cell responses might function as a natural immunoregulatory mechanism. Several recent studies have demonstrated that inducing functional IDO activity in a specific cell type can modulate GVHD by allogeneic T-cell suppression.20–22 Marrow stromal cells exhibit interferon-γ (IFN-γ)-inducible IDO activity, which contributes to allogeneic T-cell inhibition.20 In addition, exposure of dendritic cells to histone deacetylase inhibitors21 and thymosin-α122 suppresses GVHD responses in an IDO-dependent manner.
In this study, we investigated the relevance of IDO in the G-CSF-induced immature myeloid cell (gMC)-mediated suppression of alloreactive donor T cells. We found that gMCs have potent suppressive activity, resulting in the prevention of acute GVHD lethality. Although these cells acquired a considerable amount of IDO expression capacity after exposure to IFN-γ, it was not relevant in prolonging survival. Our results demonstrate that gMCs prevent lethal acute GVHD through an IDO-independent mechanism.
Materials and methods
Mice
Female C57BL/6 (H-2b), B6D2F1 (H-2b/d) and BALB/c (H-2d) mice were purchased from Charles River (Tokyo, Japan); IDO−/− C57BL/6 mice were obtained from Jackson Laboratory (Bar Harbor, ME). Mice were housed in microisolator cages at the Inje University College of Medicine. Mice used for experiments were 8–10 weeks of age. All experiments were reviewed and approved by the Institutional Animal Care and Use Committee.
Antibodies and reagents
The following antibodies, purchased from e-Bioscience (San Diego, CA), were used for phenotypic analyses: fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD11c (N418), CD45R/B220 (RA3-6B2), CD80 (16-10A1), CD86 (GL1), F4/80 (BM8), Gr-1 (RB6.8C5), major histocompatibility complex (MHC) class I (28-14-8), MHC class II (M5/114.15.2), immunoglobulin G2a (IgG2a) and IgG2b isotype control; phycoerythrin (PE)-conjugated anti-mouse CD4 (GK1.5), CD8 (53-6.7) and CD11b (M1/70); and PE-Cy5-conjugated anti-mouse Gr-1 (RB6.8C5). The FITC-conjugated anti-mouse CD68 was purchased from Serotec (Oxford, UK). The PE-conjugated anti-mouse Ly-6C (AL-21), H-2Kb (AF6-88.5) and purified anti-CD16/32 (2.4G2) were obtained from BD Biosciences (San Jose, CA). 1-Methyl-dl-tryptophan (1-MT) was purchased from Sigma-Aldrich (St Louis, MO). Recombinant human G-CSF was provided by Jeil-Kirin Pharm. Inc. (Grasin; Tokyo, Japan).
Treatment with G-CSF and isolation of immature myeloid cells
Wild-type (WT) and IDO−/− B6 donor mice were subcutaneously injected with recombinant human G-CSF (10 μg) daily for 5 days. The spleen was harvested 3 hr after the final G-CSF injection, cut into small pieces and incubated with collagenase type II (1 mg/ml; Sigma) and DNase I (15 μg/ml) at 37° for 40 min. A single-cell suspension was obtained using a cell strainer (Falcon, San Jose, CA). Cells were incubated with anti-CD90, anti-CD19 and anti-c-kit magnetic beads (Miltenyi Biotec, Auburn, CA), and additional selection was carried out on magnetic antibody cell sorting (MACS) columns to deplete T cells, B cells and haematopoietic progenitor cells. Negatively selected cells were incubated with biotin-conjugated anti-Ly6c and added to anti-biotin magnetic beads to separate Gr-1+ CD11b+ Ly6c+ gMCs. Bone-marrow derived myeloid cells (bmMCs) were prepared from the tibia and femur of naïve B6 mice using the same procedure. The purity of isolated cells was 95–97%.
Cell surface phenotype analysis
Cells were isolated from spleen or bone marrow and washed with FACS buffer [phosphate-buffered saline (PBS) containing 1% fetal calf serum and 0·05% NaN3]. Cells were preincubated with anti-CD16/32 (2.4G2) for 10 min at 4° to block non-specific binding to Fc receptors. To identify the G-CSF-induced cell population, cells were stained with FITC-anti-Gr-1 and PE-anti-CD11b or FITC-anti-lineage markers (CD3, CD45R/B220, CD11b, Gr-1 and TER119) and PE-anti-c-kit. To analyse the phenotype of the Gr-1+ CD11b+ cells, the cells were stained with PE-Cy5-anti-Gr-1, PE-anti-CD11b and FITC-anti-H-2b, anti-I-Ab anti-CD11c, anti-B220, anti-CD68, anti-F4/80 or anti-Ly6c. Fluorescence was measured using a FACScalibur (BD Bioscience), and data analysis was performed using cellquest pro software (BD Bioscience).
Mixed lymphocyte reaction (MLR)
Responder T cells were isolated from the spleen and lymph nodes of naïve B6 mice using anti-CD90 microbeads (Miltenyi Biotec). The cells (1 × 107) were suspended in PBS and incubated with 5 μm carboxyfluorescein succinimidyl ester (Invitrogen, Carlsbad, CA) for 8 min at room temperature, and the reaction was stopped with cold 5% fetal bovine serum/PBS. After washing, the cells were suspended in RPMI-1640 tissue culture medium supplemented with 10% fetal bovine serum (Invitrogen), 100 U/ml penicillin (Cambrex, East Rutherford, NJ) and 100 μg/ml streptomycin (Cambrex). Responder cells were cocultured with irradiated BALB/c splenic stimulator cells (105) in a U-bottomed 96-well plate (Costar, Corning, NY) for 4 days. To analyse the suppressive activity, MLRs were performed using coculture with no, 103, or 104 gMCs or bmMCs in the presence or absence of 250 μm 1-MT.
GVHD induction
Donor T cells were purified from the spleens of naïve B6 mice using an anti-CD90 microbead separation system (Miltenyi Biotec). The B6D2F1 recipient mice were given two separate doses of radiation (950 cGy) within 3 hr to minimize the degree of gastrointestinal toxicity. T cells (2 × 106) were transplanted into recipients via tail vein injection. Recipients were monitored daily for survival. To assess the suppressive activity, gMCs or bmMCs were isolated and cotransferred with donor T cells into irradiated recipient mice. Administration of 1-MT by oral gavage was performed as described previously.23 Either 1-MT or control vehicle was administered to recipient mice by oral gavage at a dose of 400 mg/kg twice a day from day − 1 until day 4 after the cells were transferred.
Reverse trasncription and real-time polymerase chain reactions
Total RNA was obtained from fresh isolated or IFN-γ-stimulated (using 200 U/ml IFN-γ) gMCs and bmMCs using Trizol reagent (Invitrogen). RNA was reverse-transcribed into complementary DNA (cDNA) using a polymerase chain reaction (PCR) cDNA synthesis kit (Clontech, Palo Alto, CA). The PCR was performed using sense/antisense primers. The PCR primer sequences were as follows: mouse IDO forward, 5′-GAACCGAGGGGATGACGATGT-3′ and reverse, 5′-TCGTGCAGTGCCTTTTCCAA-3′; mouse GAPDH forward, 5′-TTCACCACCATGGAGA. AGGC-3′ and reverse, 5′-GGCATGGACTGTGGTCATGA-3′. Real-time PCR, using a SYBR-green Supermix (Bio-Rad, Hercules, CA), were performed on the iCycler System (Bio-Rad), according to the manufacturer’s instructions.
IDO enzyme activity
Kynurenine concentrations in cell culture supernatant were quantified using liquid chromatography/tandem mass spectrometry (LC/MS/MS; QTrap4000; Applied Biosystems, Foster City, CA). In brief, 50 μl aliquots of sample were extracted by the addition of 50 μl acetonitrile on ice. After centrifugation (10 000 g for 5 min at 4°), aliquots of the supernatant were analysed by LC/MS/MS. The analytes were separated on a reversed-phase column (Luna C18, 2 mm inner diameter × 30 mm, 3 μm particle size; Phenomenex, Torrance, CA) with an isocratic mobile phase consisting of acetonitrile and water (30 : 70, volume/volume) containing 0·1% formic acid. The mobile phase was eluted using an Agilent 1100 series pump (Agilent, Wilmington, DE) at 0·2 ml/min. Quantification was performed by multiple reactions monitoring (MRM) of the protonated precursor ion and the related product ion for kynurenine using the external standard method. The analytical data were processed by analyst software (version 1.4.1; Applied Biosystems).
Statistics
The Kaplan–Meier product was used to obtain the survival curves. Survival data were analysed by the log-rank test. The Student’s t-test was used for the statistical analyses of the in vitro data. P< 0·05 was defined as statistically significant.
Results
Characterization of G-CSF-induced Gr-1+ CD11b+ myeloid cells
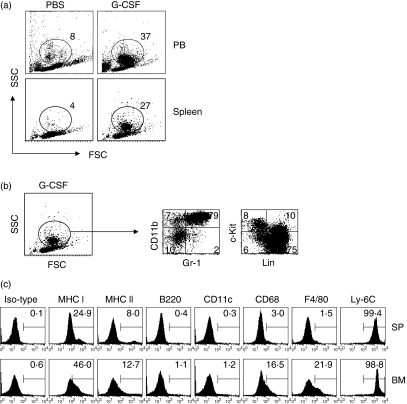
Although the regulatory properties of G-CSF-mobilized donor graft cells on GVHD have been reported, their character is still unclear. We found a marked expansion of new cell populations in peripheral blood (PB) and spleen from G-CSF-injected mice (38·4 ± 5·6% in the total PB cells, 26·5 ± 3·2% in the total splenocytes) compared with control mice (7·1 ± 2·4% in the total PB cells, 2·9 ± 1·3% in the total splenocytes) (Fig. 1a). Because G-CSF affects the mobilization of haematopoietic progenitor cells and the myeloid cell lineages from bone marrow into peripheral blood, we next analysed the expression of particular surface markers of these cell types. As shown in Fig. 1(b), approximately 80% of G-CSF-induced cells were Gr-1+ CD11b+ cells. In the other subpopulations, 10% were CD11b+ Gr-1− monocytes and 10% were lineage− c-kit+ haematopoietic progenitor cells.
Figure 1.
Phenotypic characteristics of granulocyte colony-stimulating factor (G-CSF)-induced immature myeloid cells (gMCs). Naïve B6 mice were injected subcutaneously with control phosphate-buffered saline (PBS) or G-CSF (10 μg) daily for 5 days. Peripheral blood cells (PBCs) and plenocytes were obtained and the subpopulation of cells was analysed by flow cytometry. (a) The dot plot analysis shows the percentage of gated cells analysed for forward-scatter (FSC) and side-scatter (SSC) properties. (b) Splenocytes were obtained from G-CSF-injected B6 mice and stained with fluorscein isothiocyanate (FITC)-anti-Gr-1 and phycoerythrin (PE)-anti-CD11b or FITC-anti-linage markers and PE-anti-c-kit. Data represent the percentage of quadrant gated on R1. (c) Splenocytes (SP) and bone marrow cells (BM) were obtained from G-CSF-injected and naïve B6 mice, respectively. Cells were stained for Gr-1, CD11b, and each surface marker was investigated by three-colour fluorescence. The histogram shows the percentage of positive cells gated from the Gr-1+ CD11b+ cells. Representative data from one of three experiments are shown; all three experiments showed similar results.
To determine the phenotype of G-CSF-induced Gr-1+ CD11b+ cells, we compared the expression of surface markers in these cells with that in the same marker-positive cells from naïve bone marrow (Fig. 1c). The G-CSF-induced Gr-1+ CD11b+ cells expressed the immature myeloid cell marker Ly6c but did not express mature monocyte/macrophage markers, including CD68, F4/80 and MHC class II. In addition, they did not express the dendritic cell markers CD11c and B220. The cellular phenotype is similar to that of bone marrow Gr-1+ CD11b+ cells. These data indicate that the G-CSF injection-induced cell population in peripheral organs is composed of Gr-1+ CD11b+ cells whose phenotype is consistent with immature myeloid cells.
gMCs suppress alloreactive donor T cells, resulting in the inhibition of acute GVHD lethality
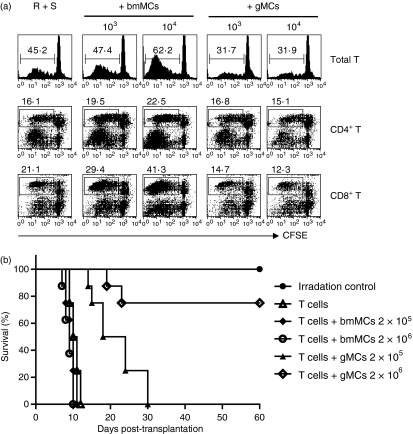
To assess the suppressive effect of G-CSF-induced Gr-1+ CD11b+ immature myeloid cells (gMCs) on alloreactive T-cell responses, we compared gMCs with bone marrow Gr-1+ CD11b+ myeloid cells (bmMCs) on the allogeneic MLRs. As shown in Fig. 2(a), bmMCs significantly enhanced alloreactive T-cell expansion in a cell number-dependent manner (103, 47·1 ± 3·2%; 104, 63·3 ± 3·5%). Both alloreactive CD4+ T cells (103, 20·8 ± 2·2%; 104, 23·1 ± 2·8%) and CD8+ T cells (103, 28·6 ± 2·1%; 104, 41·6 ± 3·2%) were stimulated with bmMCs. In contrast, gMCs significantly inhibited alloreactive T-cell expansion (103, 29·6 ± 3·1%; 104, 30·6 ± 2·2%). The suppression was more sensitive with CD8+ T cells (103, 15·2 ± 2·4%; 104, 11·6 ± 2·3%) than with CD4+ T cells (103, 17·7 ± 2·5%; 104, 15·3 ± 2·8%).
Figure 2.
Granulocyte colony-stimulating factor (G-CSF)-induced immature myeloid cells (gMCs) suppress alloreactive T-cell expansion, which results in inhibition of acute graft-versus-host disease (GVHD) lethality. (a) Mixed lymphocyte reaction (MLR) cultures were set up with 105 carboxyfluorescein succinimidyl ester-labelled naïve B6 T-cell responder (R) and 105 BALB/c splenic stimulators (S) per well. Both gMCs and bone marrow-derived myeloid cells (bmMCs) were isolated from the spleens of G-CSF-injected mice and the bone marrow of naïve B6 mice, respectively. MLRs were cocultured with no, 103 or 104 gMCs or bmMCs for 4 days. Cultures were harvested and stained with PE-Cy5-anti-H-2Kb with or without PE-anti-CD4 or CD8, and analysed by flow cytometry. (b) B6D2F1 recipient mice were lethally irradiated (950 cGy) and transferred with 2 × 106 B6 donor T cells alone or plus each above-listed number of gMCs or bmMCs. Each group consisted of eight mice. Data represent the results from three similar experiments. *P < 0·05, gMCs versus bmMCs.
We next addressed whether adoptive transfer of gMCs could prevent acute GVHD by using a well-characterized murine allogeneic bone marrow transplantation model (B6 → B6D2F1). The recipients that were cotransferred with donor T cells and bmMCs developed an enhanced acute GVHD lethality compared with donor T cells alone. In contrast, adoptive transfer of gMCs to allogeneic recipients significantly improved the survival rate from acute GVHD in a cell number-dependent manner (Fig. 2b). These data suggest that gMCs have strong tolerogenic properties that can inhibit acute GVHD.
Tryptophan catabolism in gMCs
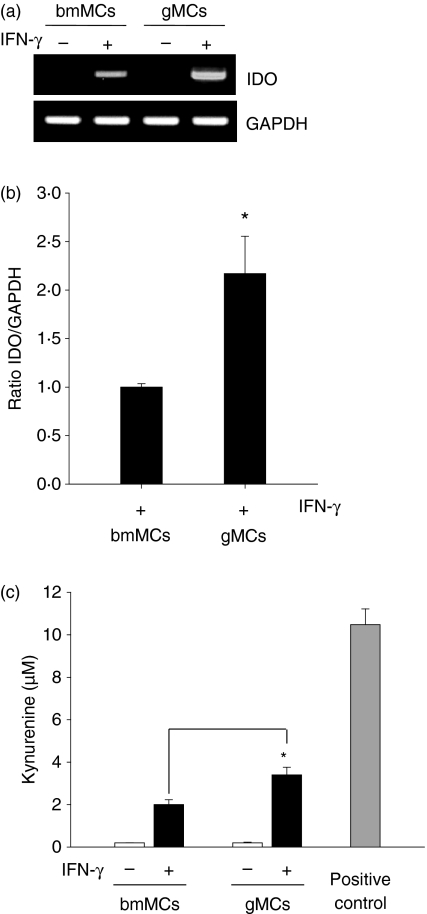
To determine whether tryptophan catabolism is associated with the tolerogenic properties of gMCs, we analysed IDO expression using reverse trasncription (RT)-PCR. The IDO messenger RNA was not detected in either gMCs or bmMCs (Fig. 3a), suggesting that G-CSF signalling does not directly induce IDO expression in myeloid cells.
Figure 3.
Indoleamine 2,3-dioxygenase (IDO) is induced in granulocyte colony-stimulating factor (G-CSF)-induced immature myeloid cells (gMCs) following interferon-γ (IFN-γ) treatment. (a) The gMCs and bone-marrow-derived myeloid cells (bmMCs) were isolated from the spleens of G-CSF-injected mice or the bone marrow of naïve B6 mice as described in the Materials and methods. Total RNA was extracted from freshly isolated cells (−) and reverse transcribed into complementary DNA. Polymerase chain reaction (PCR) was performed using IDO-specific primers, and the products were analysed by agarose gel electrophoresis. Isolated cells were stimulated with 200 U/ml IFN-γ for 24 hr (+). (b) IFN-γ-induced IDO expression levels were compared between gMCs and bmMCs using real-time PCR. Results are expressed as the mean ratio of IDO : GAPDH ± SD. *P < 0·05, gMCs versus bmMCs. (c) Cells were cultured in the absence (−) or presence (+) of IFN-γ. After 2 days of culture, the supernatant was harvested and the kynurenine concentration was measured by high-performance liquid chromatography as described in the Materials and methods. The positive control (PC) was prepared from IFN-γ-treated RAW cells. Levels are expressed as μm± SD. *P < 0·05, gMCs versus bmMCs.
The IDO is essentially induced in various cell types by IFN-γ.24–26 Indeed, IFN-γ is largely produced in the recipient by alloreactive donor T cells after allo-HSCT. Therefore, we next tested whether gMCs could acquire the capacity of robust IDO expression in response to IFN-γ. As shown in Fig. 3(a), strong IDO expression was detected following treatment of both gMCs and bmMCs with IFN-γ. However, the expression level in gMCs was significantly higher than in bmMCs. Real-time PCR analysis showed twofold higher levels of IDO messenger RNA in gMCs compared with bmMCs (P < 0·05, Fig. 3b). Enzyme activity showed a similar pattern with messenger RNA expression, which was significantly higher in gMCs than in bmMCs (P < 0·05, Fig. 3b). These results indicate that IDO is not directly induced in gMCs by G-CSF signalling. However, G-CSF does increase the capacity for robust IDO expression in response to IFN-γ.
Effect of IDO on gMC-mediated alloreactive T-cell suppression
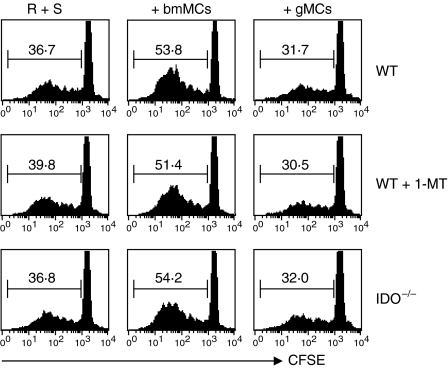
To address whether IDO is critical to the suppressive function of gMCs on alloreactive T-cell expansion, we performed the allo-MLRs using a pharmacological inhibitor of IDO, 1-MT. Treatment with 1-MT did not reverse the alloreactive T-cell suppression by gMCs (29·6 ± 3·1 in the 1-MT treated group versus 30·1 ± 2·6 in the control treated group) (Fig. 4). To confirm the results, we isolated gMCs from G-CSF-injected WT and IDO−/− mice, respectively, and then cocultured in MLRs. The suppression rate of alloreactive T-cell expansion was still maintained by IDO−/− gMCs (30·9 ± 2·4), which are similar to WT gMCs (Fig. 4). These data indicate that IDO expression in gMCs may not be critical for gMC suppression of alloreactive donor T cells in MLRs.
Figure 4.
Effect of indoleamine 2,3-dioxygenase (IDO) on granulocyte colony-stimulating factor (G-CSF)-induced immature myeloid cell (gMC)-mediated alloreactive T-cell suppression. Mixed lymphocyte reaction (MLR) cultures were set up as described in Fig. 2a (upper histogram). MLRs alone, MLRs/gMCs (104) or MLRs/bone-marrow derived myeloid cells (bmMCs; 104) were cultured in the presence of 1-methyl-dl-tryptophan (1-MT; 250 μm) or control vehicle (middle histogram). The gMCs and bmMCs were isolated from the spleens of G-CSF-injected mice and the bone marrow of naive IDO−/− B6 mice, respectively and cocultured in MLRs (low histogram). Representative data from one experiment of three are shown.
Effect of IDO on gMC-mediated inhibition of acute GVHD lethality
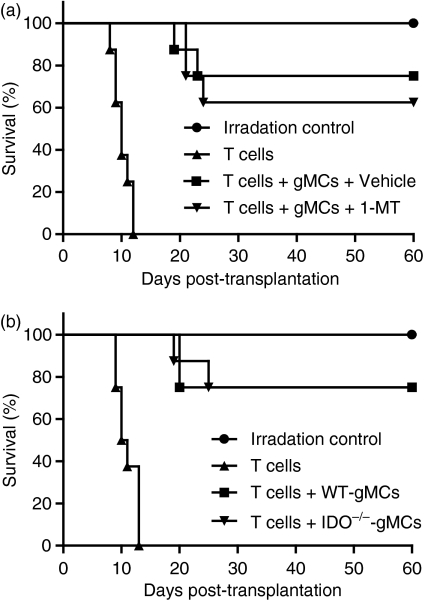
To directly address whether IDO is associated with gMC-mediated inhibition of acute GVHD lethality, we administered 1-MT to the recipients that were cotransferred with donor T cells and gMCs. However, there was no difference in the survival rate between the 1-MT-treated and control vehicle-treated recipients (Fig. 5a). We also observed that the recipients that received an adoptive transfer with IDO−/− gMCs had a survival rate similar to that of WT gMCs (Fig. 5b). Taken together, these data indicate that tryptophan catabolism is not associated with gMC-mediated inhibition of lethal acute GVHD.
Figure 5.
Effect of indoleamine 2,3-dioxygenase (IDO) on granulocyte colony-stimulating factor (G-CSF)-induced immature myeloid cell (gMC)-mediated inhibition of acute graft-versus-host disease (GVHD). (a) B6D2F1-recipient mice were lethally irradiated (950 cGy) and transferred with 2 × 106 B6 donor T cells alone or plus 2 × 106 gMC. Recipient mice were orally administered with 1-methyl-dl-tryptophan (1-MT) or control vehicle daily from day − 1 until 4 days after the cells were transferred. (b) The gMCs were isolated from G-CSF-injected wild-type (WT) and IDO−/− mice and cotransferred with donor T cells to irradiated recipient mice. Each group consisted of eight mice. Data represent the results from two similar experiments.
Discussion
Numerous studies have revealed the regulatory effects of G-CSF in allo-HSCT in both humans and mice.9,10 In the mouse system, when splenocytes from G-CSF-injected mice were transplanted, the recipients were completely protected from developing lethal acute GVHD.27 For this reason, we investigated which cellular component protects recipients from acute GVHD. We have tested the cellular components of donor grafts associated with the direct and indirect regulatory pathways of G-CSF. Although a direct effect of G-CSF on donor T cells has been reported,28,29 we observed that T cells isolated from the spleens of G-CSF-injected donor mice have normal alloreactivity (data not shown). Therefore, we further focused on the indirect regulatory effects of myeloid cells in G-CSF-injected donor grafts. We found that a large number of Gr-1+ CD11b+ cells were observed in the PB and spleens of G-CSF injected donor mice (Fig. 1a,b). These cells are different from low-density granulocytes, which are CD11b negative.15 Using phenotypic analysis, we determined that these cells are immature myeloid cells that express the same markers as bone marrow Gr-1+ CD11b+ cells (Fig. 1c), suggesting that gMCs might be mobilized from the bone marrow by G-CSF treatment. However, gMCs strongly inhibited alloreactive donor T-cell expansion in MLR cultures whereas bmMCs promoted proliferation (Fig. 2a). Moreover, when gMCs were used for adoptive transfer, the recipients were significantly protected from developing acute GVHD lethality (Fig. 2b). These results demonstrate that gMCs are the cellular component of the donor graft critical for suppression of alloreactive donor T cells following G-CSF injection.
In the present study, we investigated whether IDO is responsible for gMC-mediated suppression of alloreactive donor T cells. This enzyme can be induced in antigen-presenting cells, especially tolerogenic dendritic cells, by cytokine signalling, and then it plays a critical role in the suppression of T-cell responses. It has also been reported that IDO expressing human bone marrow stromal cells inhibit allogeneic T-cell responses.20 Dendritic cells exposed to histone deacetylase inhibitors are induced by IDO to reduce GVHD.21 These reports indicate that IDO plays an important role in regulatory antigen-presenting cells to regulate alloreactive donor T cells after allo-HSCT. However, gMCs as well as bmMCs do not express IDO (Fig. 3a); IDO was not induced in bmMCs by treatment with rhG-CSF in vitro (data not shown). An early report has shown that IDO message is undetectable in macrophage colony-stimulating factor (MCSF)-derived macrophages before activation with IFN-γ.30 Our results indicate that G-CSF signalling could not directly induce IDO expression in myeloid cells.
Post-HSCT, donor-derived cells could be stimulated with various inflammatory cytokines especially IFN-γ. Although IFN-γ has pathogenic effects, it is also required for the inhibition of acute GVHD.31,32 Therefore, it is possible that the intrinsic regulatory system might be induced by IFN-γ resulting in suppression of alloreactive donor T cells. It has been reported that T-cell dysfunction after HSCT can mediate IDO augmentation in monocytes post-HSCT.33 In addition, when MCSF-derived macrophages are cultured with activated T cells, tryptophan catabolism can be induced by T-cell-derived IFN-γ, resulting in the inhibition of T-cell proliferation.30 We also observed that IDO expression and activity are markedly induced in gMCs after exposure to IFN-γ compared with bmMCs (Fig. 3b,c), demonstrating that gMCs acquire the capacity for robust IDO activity in response to IFN-γ as a post-HSCT condition. We confirmed the functional relevance for IDO in gMCs using the pharmacological inhibitor 1-MT and IDO−/− mice. However, we could not find a functional role for IDO in gMCs in inhibition of acute GVHD lethality or suppression of alloreactive T cells. When 1-MT was given after transplantation, recipients still maintained gMC-mediated survival (Fig. 5a). In addition, IDO−/− gMCs have the same suppressive effects on alloreactive T cells as WT gMCs (Fig. 4). Under conditions of adoptive transfer with IDO−/− gMCs, there was no difference among the survival recipients compared with WT gMCs (Fig. 5b). Taken together, these results suggest that IDO is dispensable for the tolerogenic properties of gMCs on acute GVHD.
Although the T-cell suppressive activity of IDO on many regulatory systems is well known, it is still controversial. Human marrow stromal cells inhibit allogeneic T-cell responses by IDO dependency.20 However, it has also been reported that the tolerogenic properties of these cells are not relevant to IDO.34 These different results suggest that there is a link between functional activity and the amount of expression. This means that sufficient amounts of IDO must be required to induce an inhibitory effect. In our experiment, the possible reason for the finding that IDO is not essential for the tolerogenic properties of gMCs may be related to the lifespan of these cells. Isolated gMCs as well as low-density granulocytes35,36 were observed undergoing apoptosis within 48 hr in vitro and in vivo (data not shown). After transplantation, donor T cells home to the target organs of acute GVHD, and then division starts on the third day.37 Generation of IFN-γ begins after donor T-cell division. Therefore, during the gMCs’ survival period in the recipient, IFN-γ levels may be insufficient to induce a functional amount of IDO activity. Further studies are needed to determine what conditions promote survival of gMCs so that the tolerogenic capacity can be augmented by induction and duration of IDO activity.
In summary, our findings show that gMCs are induced in G-CSF-injected donor graft tissue and strongly inhibit acute GVHD lethality by suppression of alloreactive T cells. These cells do not express IDO but this enzyme is induced by treatment with IFN-γ. However, they still inhibit acute GVHD lethality in the absence of IDO. In conclusion, our present study demonstrates that gMCs are a potent therapeutic target for preventing acute GVHD and that this effect is IDO-independent.
Acknowledgments
This research was supported by the 2005 Inje University research grant (S.K.S.), and by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MOST) (R13-2007-023-00000-0 to I.C.). We thank Jeil-Kirin Pharm. Inc. for providing the rhG-CSF.
References
- 1.Berger C, Bertz H, Schmoor C, et al. Influence of recombinant human granulocyte colony-stimulating factor (filgrastim) on hematopoietic recovery and outcome following allogeneic bone marrow transplantation (BMT) from volunteer unrelated donors. Bone Marrow Transplant. 1999;23:983–90. doi: 10.1038/sj.bmt.1701746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ringden O, Remberger M, Runde V, et al. Peripheral blood stem cell transplantation from unrelated donors: a comparison with marrow transplantation. Blood. 1999;94:455–64. [PubMed] [Google Scholar]
- 3.Miflin G, Russell NH, Hutchinson RM, et al. Allogeneic peripheral blood stem cell transplantation for haematological malignancies – an analysis of kinetics of engraftment and GVHD risk. Bone Marrow Transplant. 1997;19:9–13. doi: 10.1038/sj.bmt.1700603. [DOI] [PubMed] [Google Scholar]
- 4.Ringden O, Remberger M, Runde V, et al. Faster engraftment of neutrophils and platelets with peripheral blood stem cells from unrelated donors: a comparison with marrow transplantation. Bone Marrow Transplant. 2000;25(Suppl. 2):S6–8. doi: 10.1038/sj.bmt.1702343. [DOI] [PubMed] [Google Scholar]
- 5.Bensinger WI, Martin PJ, Storer B, et al. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives inpatients with hematologic cancers. N Engl J Med. 2001;344:175–81. doi: 10.1056/NEJM200101183440303. [DOI] [PubMed] [Google Scholar]
- 6.Bensinger WI, Clift R, Martin P. Allogeneic peripheral blood stem cell transplantation in patients with advanced hematologic malignancies: a retrospective comparison with marrow transplantation. Blood. 1996;88:2794–800. [PubMed] [Google Scholar]
- 7.Mohty M, Kuentz M, Michallet M, et al. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation: long-term results of a randomized study. Blood. 2002;100:3128–34. doi: 10.1182/blood.V100.9.3128. [DOI] [PubMed] [Google Scholar]
- 8.Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19:3685–91. doi: 10.1200/JCO.2001.19.16.3685. [DOI] [PubMed] [Google Scholar]
- 9.Edward SM, Kelli PAM, Geoffrey RH. Stem cell mobilization with G-CSF analogs: a rational approach to separate GVHD and GVL? Blood. 2006;107:3430–5. doi: 10.1182/blood-2005-10-4299. [DOI] [PubMed] [Google Scholar]
- 10.Rutella S, Zavala F, Danese S, Kared H, Leone G. Granulocyte colony-stimulating factor: a novel mediator of T cell tolerance. J Immunol. 2005;175:7085–91. doi: 10.4049/jimmunol.175.11.7085. [DOI] [PubMed] [Google Scholar]
- 11.Mielcarek M, Martin PJ, Torok-Storb B. Suppression of alloantigen-induced T-cell proliferation by CD14+ cells derived from granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells. Blood. 1997;89:1629–34. [PubMed] [Google Scholar]
- 12.Mielcarek M, Graf L, Johnson G, Torok-storb B. Production of interleukin-10 by granulocyte colony-stimulating factor-mobilized blood products: a mechanism for monocyte-mediated suppression of T-cell proliferation. Blood. 1998;92:215–22. [PubMed] [Google Scholar]
- 13.MacDonald KP, Rowe V, Clouston AD, et al. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–50. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 14.Zeng D, Dejbakhsh-Jones S, Strober S. Granulocyte colony-stimulating factor reduces the capacity of blood mononuclear cells to induce graft-versus-host disease: impact on blood progenitor cell transplantation. Blood. 1997;90:453–63. [PubMed] [Google Scholar]
- 15.Vasconcelos ZF, Dos Santos BM, Farache J, et al. G-CSF-treated granulocytes inhibit acute graft-versus-host disease. Blood. 2006;107:2192–9. doi: 10.1182/blood-2005-08-3239. [DOI] [PubMed] [Google Scholar]
- 16.Shimizu T, Nomiyama S, Hirata F, Hayaishi O. Indoleamine 2,3-dioxygenase: purification and some properties. J Biol Chem. 1978;253:4700–6. [PubMed] [Google Scholar]
- 17.Taylor MW, Feng G. Relationship between IFN-, indoleamine 2,3-dioxygenase, and tryptophan catabolism. FASEB J. 1991;5:2516–22. [PubMed] [Google Scholar]
- 18.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–74. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 19.Munn DH, Zhou M, Attwood JT, et al. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–3. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- 20.Meisel R, Zibert A, Laryea M, Göbel U, Däubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103:4619–21. doi: 10.1182/blood-2003-11-3909. [DOI] [PubMed] [Google Scholar]
- 21.Reddy P, Sun Y, Toubai T, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–73. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Romani L, Bistoni F, Perruccio K, et al. Thymosin α1 activates dendritic cell tryptophan catabolism and establishes a regulatory environment for balance of inflammation and tolerance. Blood. 2006;108:2265–74. doi: 10.1182/blood-2006-02-004762. [DOI] [PubMed] [Google Scholar]
- 23.Hou DY, Muller AJ, Sharma MD, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methy-tryptophan correlated with antitumor responses. Cancer Res. 2007;67:792–801. doi: 10.1158/0008-5472.CAN-06-2925. [DOI] [PubMed] [Google Scholar]
- 24.Takikawa O, Kuroiwa T, Yamazaki F, Kido R. Mechanism of interferon-γ action: characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-γ and evaluation of the enzyme-mediated tryptophan degradation in anticellular activity. J Biol Chem. 1998;263:2041–8. [PubMed] [Google Scholar]
- 25.Hwu P, Du MX, Lapointe R, et al. Indoleamine-2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–9. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- 26.Puccetti P. On watching the watchers: IDO and type I/II IFN. Eur J Immunol. 2007;37:876–9. doi: 10.1002/eji.200737184. [DOI] [PubMed] [Google Scholar]
- 27.Pan L, Teshima T, Hill GR, et al. Granulocyte colony-stimulating factor-mobilized allogeneic stem cell transplantation maintains graft-versus-leukemia effects through a perforin-dependent pathway while preventing graft-versus-host disease. Blood. 1999;93:4071–8. [PubMed] [Google Scholar]
- 28.Pan L, Delmonte JJ, Jalonen CK, Ferrara JL. Pretreatment of donor mice with granulocyte colony-stimulating factor polarizes donor T lymphocytes toward type-2 cytokine production and reduces severity of experimental graft-versus-host disease. Blood. 1995;86:4422–9. [PubMed] [Google Scholar]
- 29.Franzke A, Piao W, Lauber J, et al. G-CSF as immune regulator in T cells expressing the G-CSF receptor: implications for transplantation and autoimmune diseases. Blood. 2003;102:734–9. doi: 10.1182/blood-2002-04-1200. [DOI] [PubMed] [Google Scholar]
- 30.Munn DH, Shafizadeh E, Attwood JT, Bondarev I, Pashine A, Mellor AL. Inhibition of T cell proliferation by macrophage tryptophan catabolism. J Exp Med. 1993;189:1363–72. doi: 10.1084/jem.189.9.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang YG, Dey BR, Sergio JJ, Pearson DA, Sykes M. Donor-derived interferon gamma is required for inhibition of acute graft-versus-host disease by interleukin 12. J Clin Invest. 1998;102:2126–35. doi: 10.1172/JCI4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang YG, Qi J, Wang MG, Sykes M. Donor-derived interferon gamma separates graft-versus-leukemia effects and graft-versus-host disease induced by donor CD8 T cells. Blood. 2002;99:4207–15. doi: 10.1182/blood.v99.11.4207. [DOI] [PubMed] [Google Scholar]
- 33.Hainz U, Obexer P, Winkler C, et al. Monocyte-mediated T-cell suppression and augmented monocyte tryptophan catabolism after human hematopoietic stem-cell transplantation. Blood. 2005;105:4127–34. doi: 10.1182/blood-2004-05-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gieseke F, Schutt B, Viebahn S, Koscielniak E, Friedrich W, Handgretinger R, Muller I. Human multipotent mesenchymal stromal cells inhibit proliferation of PBMCs independently of IFNγR1 signaling and IDO expression. Blood. 2007;110:2197–2200. doi: 10.1182/blood-2007-04-083162. [DOI] [PubMed] [Google Scholar]
- 35.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 36.Vasconcelos ZF, Santos BM, Costa ES. T-lymphocytes function from peripheral blood stem cell donors is inhibited by activated granulocytes. Cytotherapy. 2003;5:336–45. doi: 10.1080/14653240310002252. [DOI] [PubMed] [Google Scholar]
- 37.New JY, Li B, Koh WP, et al. T cell infiltration and chemokine expression: relevance to the disease localization in murine graft-versus-host disease. Bone Marrow Transplant. 2002;29:979–86. doi: 10.1038/sj.bmt.1703563. [DOI] [PubMed] [Google Scholar]