Abstract
The ubiquitin specific protease USP25 has been identified by the Melchior lab as a SUMO-2/3 interacting protein and substrate for SUMO modification. Specificity for SUMO-2/3 is achieved by a USP25 SUMO interacting motif, and SUMO modification at sites proximal to two ubiquitin interacting domains diminishes USP25's ability to bind and degrade poly-ubiquitin chains.
Post-translational modification of protein substrates by ubiquitin (Ub) and SUMO (a ubiquitin-like protein) contributes to signal transduction in pathways that control diverse events such as the cell cycle, apoptosis, cytokinesis, protein degradation, to name a few (Kersher et. al., 2006; Geiss-Freidlander and Melchior, 2007). Only one ubiquitin isoform exists, but a diverse array of signals can be generated by altering the topology of ubiquitin conjugates through mono- or poly-ubiquitination wherein distinct ubiquitin lysine residues are used to form unique Ub-Ub linkages. These signals can be recognized by factors containing ubiquitin recognition motifs such as UIM or UBA domains.
Only one SUMO isoform exists in lower eukaryotes, but three SUMO isoforms exist in higher eukaryotes, SUMO-1, SUMO-2, and SUMO-3. SUMO-2 and SUMO-3 are highly related (97% sequence identity; therefore referred to as SUMO-2/3 henceforth), but share only 47% sequence identity to SUMO-1. SUMO isoforms also differ in their ability to form chains. Unlike SUMO-1, SUMO-2/3 have conserved N-terminal lysine residues that can be used to form branch points in SUMO chains. These differences generate signal diversity in the SUMO pathway by incorporating unique SUMO isoforms or chains into substrates that are recognized by downstream factors containing SUMO interacting motifs (SIM) (Vertegaal, 2007).
Substrates are conjugated to distinct SUMO isoforms in vivo, however most data suggest that the SUMO conjugation apparatus is incapable of discriminating between SUMO isoforms. These observations support the existence of alternative mechanisms that enforce specificity. In this issue of Molecular Cell, Meulmeester and colleagues reveal at least one potential mechanism by which a substrate can select a particular SUMO isoform for conjugation (Meulmeester et al., 2008).
In this study, a biochemical screen for SUMO-1 and SUMO-3 binding proteins identified the deubiquitinating (DUB) enzyme family member USP25 as a SUMO interacting protein and substrate for SUMO modification. USP25 interacted with both SUMO-1 and SUMO-3, but it exhibited preference for SUMO-3. A SIM near the USP25 N-terminus was essential for SUMO interaction and SIM-mediated recruitment of the E2-SUMO-3 thioester promoted E3-independent conjugation to at least two non-consensus USP25 lysine residues within and proximal to the USP25 UIM domains. High SUMO-3 concentrations were required to compete for E2-SUMO-3-mediated USP25 conjugation, so while SIM-SUMO-3 interactions were necessary, data suggests that USP25 also interacts with the SUMO E2 Ubc9. USP25 SIM-SUMO interactions were required to achieve specificity, but it is interesting to note that SIM elements remained functional after being transposed out of their native structural context to the USP25 N-terminus.
While the biological function for USP25 is currently unknown, biochemical characterization revealed that USP25 was more efficient in hydrolyzing K48 and K63 linked poly-ubiquitin chains when compared to processing ubiquitin-AMC. Furthermore, deletion analysis suggested that the two USP25 UIM elements were required for binding poly-ubiquitin chains and for full activity during poly-ubiquitin chain degradation. So what affect does SUMO-3 modification have on USP25 function? The USP25 SUMO-3 conjugate maintained the ability to hydrolyze ubiquitin-AMC, but it exhibited lower activities during poly-ubiquitin chain degradation. This reduction in activity correlated well with weakened interactions between SUMO-modified USP25 and poly-ubiquitin. Two simple mechanisms could account for these data. The first suggests that SUMO modification blocks the UIM binding surfaces independent of any USP25 conformational change. The second suggests that SIM-SUMO interactions are maintained to stabilize a USP25 conformational change that blocks UIM-ubiquitin interactions (Figure 1B). This latter model is conceptually similar to one proposed for mono-ubiquitinated substrates such as Sts1 and Eps15 (Hoeller et. al., 2006). Further characterization of SUMO-3 modified USP25 will be required to validate either of these models.
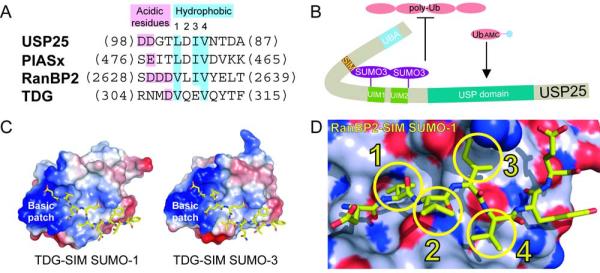
Figure 1. SUMO interacting motifs.
A) Sequence alignment for SIM elements from USP25, PIASx, RanBP2, and TDG. The latter three SIM elements are aligned based on structure. USP25 was aligned to PIASx since it exhibited the highest similarity. Acidic and hydrophobic elements are labeled and color-coded. B) A speculative model for SUMO-3-USP25 interactions wherein the SIM maintains interactions with the most N-terminal SUMO-3. This model could explain why catalytic activities are lost for polyubiquitin chains but maintained for hydrolysis of ubiquitin-AMC. C) Surface and electrostatic representations for SUMO-1 and SUMO-3 in complex with TDG SIM elements shown in yellow stick representation to highlight the basic patch (PDB 1WYW and 2D07). D) Surface representation for SUMO-1 indicating nitrogen (dark blue), oxygen (red), or carbon (light blue) in complex with the SIM from RanBP2/Nup358 colored in yellow stick representation (PDB 1Z5S). The four hydrophobic SIM amino acids are highlighted with circles and numbered 1-4 corresponding to panel A.
While USP25 preferred SUMO-2/3, it still interacted with SUMO-1, so the molecular basis for SIM-mediated discrimination remains somewhat unclear. Comparing various SIM-SUMO complexes reveals few clues as to how SIMs might establish isoform specificity (Figure 1). All SIM-SUMO complexes utilize interactions between the exposed SUMO β-sheet and SIM β-strand through main chain contacts that appear nearly identical between SUMO isoforms (Capili and Lima, 2007). The acidic SIM residues could provide specificity, but corresponding basic patches on SUMO-1 and SUMO-3 appear quite similar (Figure 1C; Baba et al., 2006).
Variations do exist between SUMO isoforms for surface pockets that engage the four hydrophobic SIM residues as exemplified by RanBP2/Nup358 (Figure 1D; Reverter and Lima, 2005), and it is important to note that these SIM positions are quite variable when SIMs are compared (Figure 1A). Unlike the RanBP2/Nup358 SIM, the USP25 SIM includes a single acidic residue at position 2 and appears similar to PIASx, a SIM that interacts with SUMO in an opposite orientation to that observed for RanBP2 or TDG (Figure 1A; Song et al., 2005). Perhaps these differences could account for alternative binding configurations that favor or disfavor interactions with a particular SUMO isoform, a feature one must understand if SUMO-specific SIMs are to be developed to validate roles for SUMO isoforms during specific biological process. Although the present study sheds light on some of these issues, additional studies will be required to fully understand how specificity is achieved in diverse biological systems.
This study expands on another pertinent issue, the intersection of SUMO and ubiquitin pathways (Geiss-Freidlander and Melchior, 2007). In addition to USP25, SUMO modification of the ubiquitin conjugating enzyme E2-25K marks another example for a SUMO-modified ubiquitin enzyme. Other proteins modified by SUMO and ubiquitin include IKBα and PCNA. While SUMO modification of IKBα antagonizes ubiquitin conjugation, ubiquitin and SUMO modification of PCNA play distinct roles during DNA damage response and replication. The final and perhaps most surprising intersection is the recent identification of a ubiquitin E3 ligase that targets SUMO chains (Perry et al., 2008). While the aforementioned processes encompass just a few instances where SUMO and ubiquitin pathways are known to intersect, the growing list suggests that many surprises are yet to come.
REFERENCES
- Baba D, Maita N, Jee JG, Uchimura Y, Saitoh H, Sugasawa K, Hanaoka F, Tochio H, Hiroaki H, Shirakawa M. J. Mol. Biol. 2006;359:137–147. doi: 10.1016/j.jmb.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Capili AD, Lima CD. Curr Opin in Struct Biol. 2007;17:726–735. doi: 10.1016/j.sbi.2007.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss-Freidlander R, Melchior F. Nat. Rev. Mol. Cell Biol. 2007;8:947–956. doi: 10.1038/nrm2293. [DOI] [PubMed] [Google Scholar]
- Hoeller D, Crosetto N, Blagoev B, Raiborg C, Tikkanen R, Wagner S, Kowanetz K, Breitling R, Mann M, Stenmark H, Dikic I. Nat. Cell Biol. 2006;8:163–169. doi: 10.1038/ncb1354. [DOI] [PubMed] [Google Scholar]
- Kerscher O, Felberbaum R, Hochstrasser M. Annu. Rev. Cell Dev. Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- Meulmeester E, Kunze M, Hsiao HH, Urlaub H, Melchior F. Molecular Cell. 2008;30:610–619. doi: 10.1016/j.molcel.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Perry JJP, Tainer JA, Boddy MN. Trends Biochem. Sci. 2008 doi: 10.1016/j.tibs.2008.02.001. In press. doi:10.1016/j.tibs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Reverter D, Lima CD. Nature. 2005;435:687–692. doi: 10.1038/nature03588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Zhang Z, Hu W, Chen Y. J. Biol. Chem. 2005;280:40122–40129. doi: 10.1074/jbc.M507059200. [DOI] [PubMed] [Google Scholar]
- Vertegaal AC. Biochem. Soc. Trans. 2007;35:1422–1423. doi: 10.1042/BST0351422. [DOI] [PubMed] [Google Scholar]