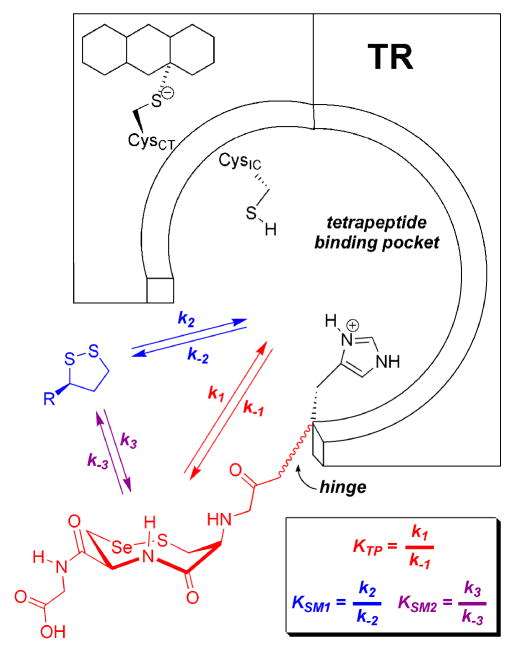
Figure 5.
Proposed model for the interaction of small molecule substrates with TR. Both the oxidized C-terminal tetrapeptide (Gly-Cys-Sec-Gly) and small molecule disulfide (such as lipoic acid) can bind in the tetrapeptide binding pocket and are thus in competitive equilibrium for interacting with the N-terminal C1VNVGC2 redox center. These equilibrium constants are represented by KSM1, for small molecules, and KTP, for the C-terminal tetrapeptide. Both of these equilibrium constants are composed of individual rate constants in the forward and reverse directions that describe the rate of reduction of either the small molecule disulfide or the C-terminal vicinal selenosulfide bond. Please note that here the 8-membered ring of the C-terminus is shown with trans amide geometry in the chair-chair conformation. Depending on the redox state of the holoenzyme, the reduction of lipoic acid can take place via the reduced C-terminal tetrapeptide (described by equilibrium constant KSM2). Thus lipoic acid can be reduced via two modes of interaction. While not proven here, the overall rate of reduction is most likely a combination of the two different pathways (and thus combination of rate constants). A similar model has been previously proposed for the reduction of DTNB (20).