Abstract
Background and Purpose
The concept of the neurovascular unit suggests that effects on brain vasculature must be considered if neuroprotection is to be achieved in stroke. We previously reported that 12/15-lipoxygenase (12/15-LOX) is upregulated in the peri-infarct area after middle cerebral artery occlusion in mice, and 12/15-LOX contributes to brain damage after ischemia–reperfusion. The current study was designed to investigate 12/15-LOX involvement in vascular injury in the ischemic brain.
Methods
In cell culture, a human brain microvascular endothelial cell line was subjected to either hypoxia or H2O2-induced oxidative stress with or without lipoxygenase inhibitors. For in vivo studies, mice were subjected to 90 minutes middle cerebral artery occlusion, and the effects of either 12/15-LOX gene knockout or treatment with lipoxygenase inhibitors were compared. Expression of 12/15-LOX and claudin-5 as well as extravasation of immunoglobulin G were detected by immunohistochemistry. Edema was measured as water content of brain hemispheres according to the wet–dry weight method.
Results
Brain endothelial cells were protected against hypoxia and H2O2 by the lipoxygenase inhibitor baicalein. After focal ischemia, 12/15-LOX was increased in neurons and endothelial cells. The vascular tight junction protein claudin-5 underwent extensive degradation in the peri-infarct area, which was partially prevented by the lipoxygenase inhibitor baicalein. Leakage of immunoglobulin G into the brain parenchyma was significantly reduced in 12/15-LOX knockout mice as well as wild-type mice treated with baicalein. Likewise, brain edema was significantly ameliorated.
Conclusion
12/15-LOX may contribute to ischemic brain damage not just by causing neuronal cell death, but also by detrimental effects on the brain microvasculature. 12/15-LOX inhibitors may thus be effective as both neuroprotectants and vasculoprotectants.
Keywords: baicalein, blood–brain barrier, edema, endothelial cell, lipoxygenase
Cerebral ischemic stress can cause blood–brain barrier (BBB) disruption and increase cerebral vascular permeability, leading to the formation of brain edema. Edema is the leading cause of cerebrovascular death within the first week after stroke,1 and oxidative stress and free radicals contribute to edema formation.2 The enzyme 12/15-lipoxygenase (12/15-LOX) is known to contribute to neuronal cell death after oxidative stress, but its involvement in BBB disruption has as yet not been studied. We previously reported 12/15-LOX to be upregulated in the peri-infarct region of the brain after transient focal ischemia in mice.3 Most of the lipoxygenase staining was neuronal, but we had also noted some endothelial staining at the time, suggesting possible effects on the brain microvasculature. The present study was designed to test if inhibition of 12/15-LOX or its genetic knockout reduces vasogenic edema related to BBB disruption after transient focal ischemia. Our findings indicate that 12/15-LOX may contribute to vascular injury and edema formation.
Materials and Methods
H2O2 Oxidative Stress in Transformed Human Brain Endothelial Cells
Transformed human brain endothelial cells4 were grown in Dulbecco medium supplemented with 10% fetal calf serum, 100 U/mL penicillin, and 100 µg/mL streptomycin at 37°C in a humidified atmosphere of 95% air and 5% CO2. Cells were seeded at 2×104 cells/500 µL/well in 48-well plates and treated after 24 hours culture. Treatment consisted of exchanging the medium to 1 mL fresh culturing medium and adding H2O2 (100, 200, or 400 µmol/L) to induce oxidative stress in the presence or absence of the lipoxygenase inhibitors 10 µmol/L baicalein (Cayman Chemicals) or 10 µmol/L AA861. The solvent DMSO (maximum 0.1% final concentration) was used as a control. To induce hypoxia, cells were kept in an atmosphere of 85% nitrogen/10% hydrogen/5% carbon dioxide at 37°C for 24 hours, whereas control cells were cultured under normal conditions. After 24 hours of treatment, medium was collected and the cells were lysed in 1 mL 0.5% Triton X-100 by incubating for 30 minutes at 37°C. Lactate dehydrogenase content was determined separately for the cell extracts and corresponding media using a Cytotoxicity Detection Kit (Roche), and the percentage of Lactate dehydrogenase released to the medium was calculated after subtracting the corresponding background value.
ALOX15 Genetic-Deficient Mice
Male ALOX15 knockout mice were bred in our animal facility and the genetically matched C57BL/6J wild-type controls were obtained from Jackson Laboratories (Bar Harbor, Maine). There were no significant neurological differences in phenotypes between the knockout mice and the wild-type mice. CD1 mice used for the pharmacological studies were obtained from Charles River Laboratories (Wilmington, Mass).
Transient Cerebral Focal Ischemia
All experiments were performed following an institutionally approved protocol in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male CD1 and either wild-type C57Bl6J or ALOX15 knockout mice approximately 12 weeks old were subjected to transient focal cerebral ischemia by intraluminal middle cerebral artery blockade with a silicon-coated nylon suture as described previously.5 Anesthesia was induced with 1% isoflurane in a mixture of 70% nitrous oxide and 30% oxygen delivered by face mask. The rectal temperature was maintained at 37±0.5°C and the regional cerebral blood flow of the right front parietal cortex was continually monitored during the surgical procedure. Under an operating microscope, the right common carotid artery and external carotid artery were exposed through a midline neck incision and its branches were electrocoagulated. An 11-mm 7-0 monofilament nylon suture coated with silicon was inserted through the proximal external carotid artery into the internal carotid artery, occluding the middle cerebral artery. After 90 minutes of middle cerebral artery occlusion (MCAO), blood flow was restored by withdrawal of the nylon suture. Blood gases and blood pressure were measured before induction of ischemia and again in ischemic mice after 1 hour of ischemia. For inhibitor studies, the lipoxygenase inhibitor, baicalein, was delivered in 300 mg/kg body weight in DMSO by intraperitoneal injection 30 minutes before the MCAO started. Control animals were injected with the equal volume DMSO.
Immunohistochemistry
Mice brains were removed at 24 hours after MCAO with the perfusion of phosphate-buffered saline (pH 7.4) and quickly frozen in liquid nitrogen. Coronal sections at the caudate level were cut on a cryostat at −20°C to 12 µm thickness and collected on glass slides. With these sections, we performed double-staining for lipoxygenase with neuronal marker, glia fibrillary-associated protein, or CD31 to investigate the expression of lipoxygenase on the neuron, astrocyte, and endothelial cells. The sections were fixed by 4% paraformaldehyde and rinsed 3 times in phosphate-buffered saline (pH 7.4). After blocking with 3% normal goat serum, sections were then incubated at 4°C overnight in a phosphate-buffered saline solution containing the primary antibodies (the affinity-purified lipoxygenase rabbit polyclonal antibody diluted 1:200 and mouse monoclonal anti-NeuN antibody [Chemicon] diluted 1:1003) in phosphate-buffered saline, 0.3% TritonX, 3% bovine serum albumin. The sections were washed and incubated for 1 hour with Alexa Fluor 555-conjugated goat antirabbit IgG antibody (Invitrogen) and Alexa Fluor 488-conjugated goat antimouse IgG antibody (Invitrogen) at a dilution of 1:500. Subsequently, the slides were covered with VECTASHIELD mounting medium with 4′,6′-diamidino-2-phenylindole (H-1200; Vector Laboratories). Double fluorescent staining of lipoxygenase with glia fibrillary-associated Protein (1:200; Chemicon) or CD31 (1:100; BD Pharmingen) and CD31 with Claudin-5 (1:100; Zymed) was performed in fresh–frozen sections.6 Signals were examined using an Olympus microscope (BX51; Olympus) equipped with fluorescein isothiocyanate conjugated and rhodamine filter set. Extravascular immunoglobulin G (IgG) was detected in fixed sections with a horseradish peroxidase-labeled antimouse IgG antibody followed by diaminobenzidine staining. The integrated density of staining was analyzed by using National Institutes of Health ImageJ software.
Water Content of the Brain
The water content of the brain tissue was measured by the wet and dry weight method as described previously.7 Briefly, cerebral tissue was divided with a blade into the right and left hemispheres along the anatomical midline. The wet weight of each hemisphere was measured using an electronic analytic balance. After drying the sample in an oven at 95°C for 5 days, the dry weight was obtained. The water content of each hemisphere was calculated using the following formula: water content (%)=100×(wet weight–dry weight)/wet weight. The contralateral hemisphere was used as a control.
Statistical Analysis
The data are expressed as mean±SD except where otherwise noted. Statistical analysis was performed using analysis of variance with Dunnett post hoc test or the Student t test. P<0.05 was considered statistically significant.
Results
Lipoxygenase Inhibitor Reduced Cell Injury in Transformed Human Brain Endothelial Cells
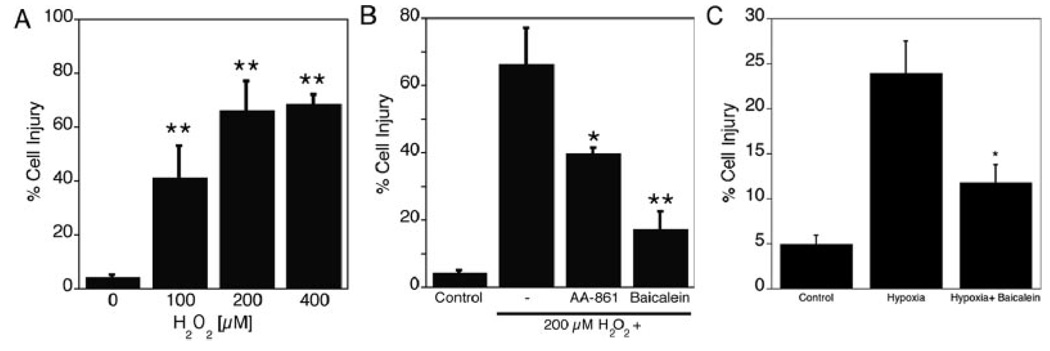
Exposure of human brain endothelial cells to 100 µmol/L, 200 µmol/L, and 400 µmol/L H2O2 for 24 hours increased the release of lactate dehydrogenase as a measure of cell injury (Figure 1A; n=4, P<0.01). Two different inhibitors of 12/15-LOX, baicalein and AA-861, both provided significant protection against 200 µmol/L H2O2 (n=3, P<0.01 and P<0.05, respectively), suggesting 12/15-LOX contributes to this form of oxidative stress in endothelial cells (Figure 1B). Likewise, subjecting the cells to 24 hours of hypoxia increased Lactate dehydrogenase release into the medium, which again was reduced by baicalein (Figure 1C).
Figure 1.
Cell injury after oxidative stress in transformed human brain endothelial (THBE) cells reduced by lipoxygenase (LOX) inhibition. Oxidative stress in THBE cells. A, A significant increase of cell injury was detected after 24 hours of treatment with H2O2 (100, 200, and 400 µmol/L), compared with control group (n=4). B, Treatment in the presence of the 12/15-LOX inhibitors baicalein or AA861 significantly protected THBE cells against 24 hours of 200 µmol/L H2O2 exposure (n=3, *P<0.05, **P<0.01). C, Cell injury after 24 hours of hypoxia was significantly reduced by treatment with 10 µmol/L baicalein (n=3, *P<0.05).
Lipoxygenase Expression in Mouse Brain Tissue
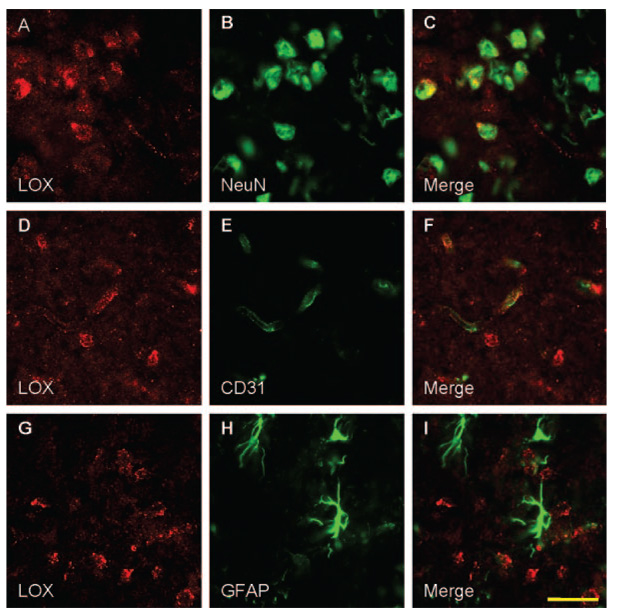
In sham control brain sections, only minimal lipoxygenase immunoreactivity was detectable (data not shown). At 24 hours after transient MCAO, increased staining for lipoxygenase was observed in the peri-ischemic area of the cerebral cortex (Figure 2A, D, G). Double immunofluorescence for lipoxygenase (red) with neuronal marker (green) showed that lipoxygenase was colocalized with the neuronal marker, as reported before (Figure 2C).3 In addition, however, colocalization of lipoxygenase (red) with the endothelial cell marker CD31 (green) was observed (Figure 2F), suggesting 12/15-LOX is also upregulated in the brain microvascular endothelium after transient focal ischemia. In contrast, lipoxygenase staining did not colocalize with glial fibril antigen protein expression (Figure 2H, green), indicating 12/15-LOX, is not upregulated to the same extent in astrocytes (Figure 2I). No immunoreactivity was found in whole brain sections when the primary antibody was omitted (data not shown).
Figure 2.
Lipoxygenase (LOX) increased in neurons and endothelial cells following transient focal ischemia. Double immunostaining for LOX (red, A, D, G) with the neuronal marker, NeuN (green, B), the endothelial cell marker CD31 (green, E), and the astrocyte marker glial fibrillary acidic protein (GFAP; green, H) in the peri-ischemic area of the cerebral cortex after 24 hours of transient MCAO. LOX expression was colocalized with the neuronal and endothelial cell markers, NeuN and CD31 (C, F), but not with the astrocytic marker GFAP (I). Scale bar: 30 µm.
Loss of Claudin-5 Protein Reduced by the Lipoxygenase Inhibitor
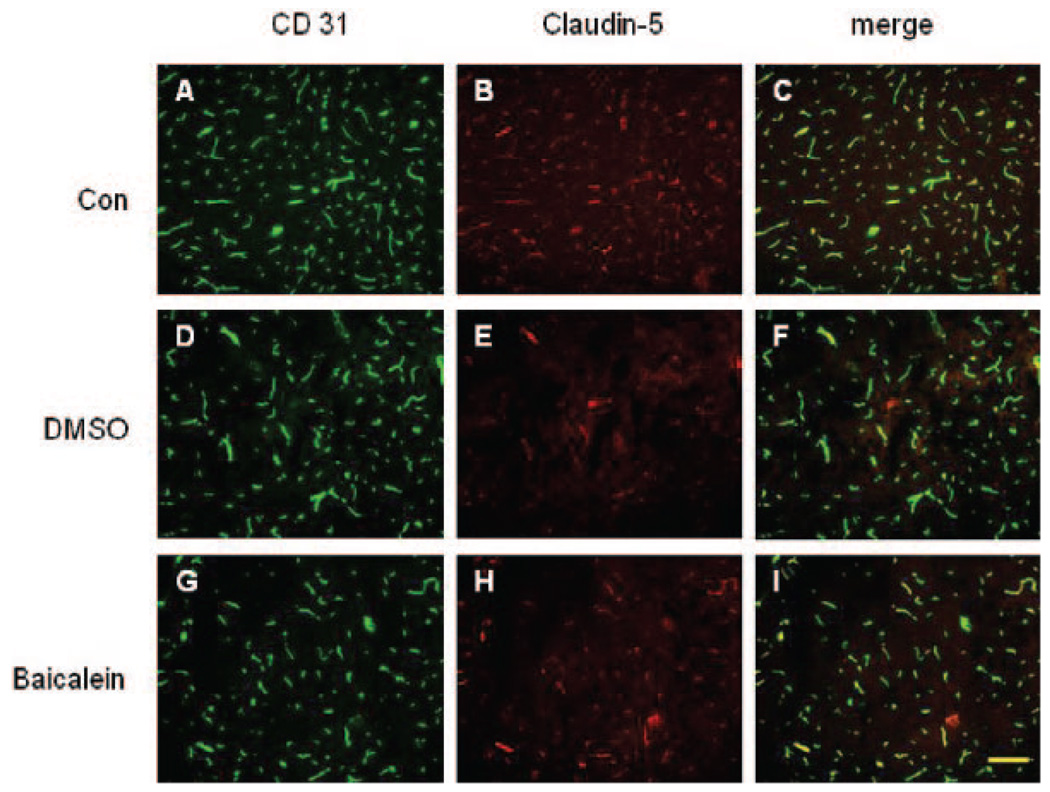
The tight junction component claudin-5 was found in blood vessels in the control brain sections (Figure 3B). With double immunofluorescence, claudin-5 colocalized with the endothelial maker CD31, as expected (Figure 3C). After focal ischemia, a clear decrease in the intensity of claudin-5 staining on the ischemic side was observed with staining absent in some vessels, suggesting that the claudin-5 was degraded (Figure 3E, 3H). However, in the peri-infarct area, claudin-5 staining was significantly better preserved in mice treated with the 12/15-LOX inhibitor baicalein (Figure 3H) compared with the DMSO-injected group (Figure 3E). No difference between treatment groups was observed for CD31 staining, indicating that the endothelial cells were still present despite loss of tight junction protein (Figure 3D, G).
Figure 3.
Protection of endothelial tight junction protein claudin-5 by lipoxygenase inhibition. Double immunostaining for CD31 (A, D, G) with claudin-5 (B, E, H) in the sham control (A–C) and in the peri-ischemic cortex of either control-treated (D–F) or baicalein-treated (G–I) animals after transient MCAO. The claudin-5 staining colocalized with the endothelial marker CD31 in these 3 groups (C, F, I), but a decrease in the intensity of claudin-5 staining was shown at 24 hours after transient MCAO (E). The loss of claudin-5 staining was significantly reduced in the baicalein treatment group (H) without significant difference in CD31 staining. Scale bar: 50 µm.
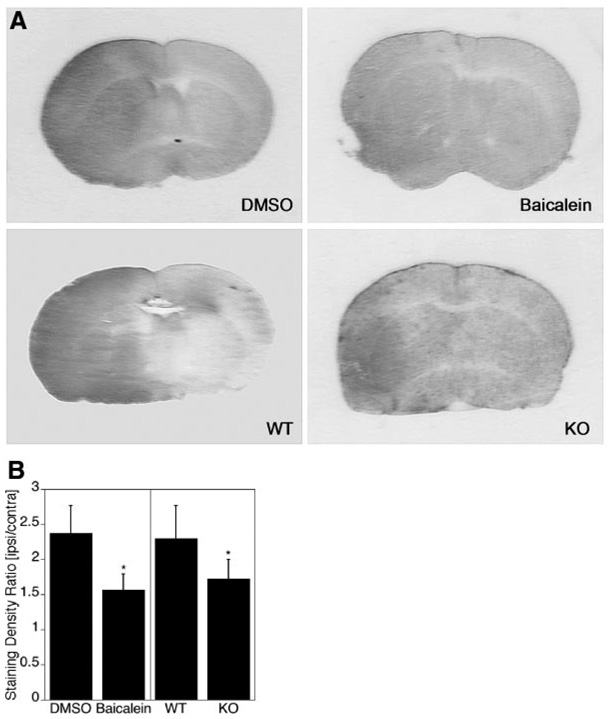
Reduced Leakage of Immunoglobulin G Into the Brain Parenchyma in the Presence of Lipoxygenase Inhibitor and in ALOX15 Knockout Mice
Physiological parameters such as pH, pO2, pCO2, and blood pressure were not significantly different between groups of wild-type mice with and without baicalein treatment and in ALOX15 knockout versus wild-type mice (Table). As a marker for BBB permeability, extravasation of IgG into the brain parenchyma was detected by immunohistochemistry. 8–10 Increased staining for IgG was detected in the ischemic hemisphere at 24 hours after 90 minutes ischemia (Figure 4A). The staining intensity was significantly reduced by either baicalein treatment or ALOX15 gene knockout (Figure 4).
Table.
Physiological Parameters*
| Pre-MCAO | Post-MCAO | |||||||
|---|---|---|---|---|---|---|---|---|
| pO2 | pCO2 | pH | Blood Pressure | pO2 | pCO2 | pH | Blood Pressure | |
| Vehicle | 169.2±24.2 | 28.8±6.1 | 7.42±0.01 | 97.8±6.0 | 139.7±22.4 | 39.1±8.1 | 7.34±0.06 | 100.4±2.1 |
| Baicalein | 153.8±18.8 | 30.6±6.6 | 7.42±0.08 | 90.5±5.3 | 136.1±17.1 | 45.0±3.8 | 7.26±0.05 | 91.0±13.8 |
| Wild-type | 172.7±12.6 | 31.6±2.4 | 7.38±0.04 | 91.3±11.0 | 136.1±12.0 | 50.5±2.3 | 7.25±0.04 | 92.5±2.9 |
| ALOX15(−/−) | 140.9±19.7 | 35.5±3.6 | 7.35±0.04 | 82.2±12.2 | 118.9±13.3 | 42.6±5.5 | 7.32±0.02 | 95.7±7.8 |
Blood gas parameters before and after 1 hour after MCAO in vehicle-treated, baicalein-treated, ALOX15(+/+), and ALOX15(−/−) groups (n=4; mean±SD).
Figure 4.
Reduced extravasation of immunoglobulin G (IgG) through either lipoxygenase (LOX) inhibition or LOX gene knockout after transient focal ischemia. A, IgG staining was significantly reduced at 24 hours after transient MCAO in the group with baicalein treatment compared with control (compare baicalein with DMSO) as well as in ALOX15 knockout mice compared with wild-type mice (compare knockout with wild-type). B, A comparison of the integrated staining densities confirms the reduction in IgG staining, suggesting that baicalein treatment or ALOX15 gene knockout protects the BBB (*P<0.05, n=6).
Ischemic Brain Edema is Reduced by Lipoxygenase Inhibitor and in ALOX15 Knockout Mice
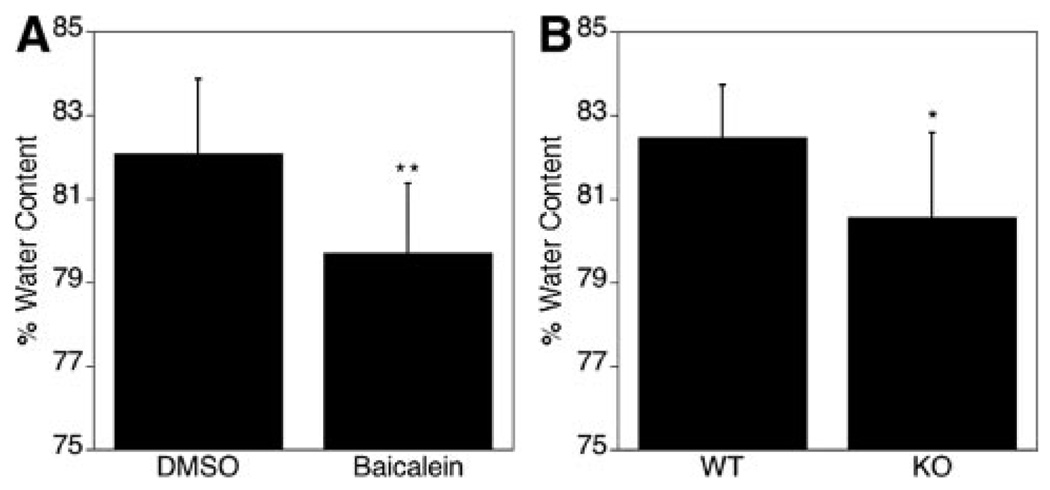
The wet–dry brain water content method was used to compare brain edema in mice subjected to focal cerebral ischemia. At 24 hours after 90 minutes ischemia, the water content was clearly elevated on the ischemic side of the brain in control-treated mice (82.1±1.77%, P<0.01, n=9). Baicalein treatment led to a significant reduction in water content (79.7±1.66%; Figure 5A). Likewise, ALOX15 knockout mice had significantly reduced water content compared with wild-type mice (80.6±2.02% versus 82.5±1.24%, P<0.05, n=8; Figure 5B). These findings suggest that the absence or inhibition of 12/15-LOX protects the brain against ischemic edema formation.
Figure 5.
Reduced edema formation through either lipoxygenase (LOX) inhibition or LOX gene knockout after transient focal ischemia. A, The water content in the brain was greatly reduced in the group with baicalein treatment at 24 hours after transient MCAO (**P<0.01, n=9). B, ALOX15 knockout mice had significantly reduced water content in the brain compared with wild-type mice (*P<0.05, n=8).
Discussion
Edema is a major complication associated with unfavorable outcome after ischemic stroke11–13 and subarachnoid hemorrhage. 14 Reducing edema by protecting the BBB can thus be seen as one aspect of rescuing the neurovascular unit, a major objective in neuroprotection.15 Oxidative stress contributes to weakening of the BBB, and reducing oxidative stress is thus seen as a valid target in the acute phase of stroke.16
It has long been known that 12/15-LOX is detrimental to neurons subjected to oxidative stress.17 We show here that brain endothelial cells can also be protected against oxidative stress produced either directly by hydrogen peroxide treatment or indirectly through hypoxia in vitro by lipoxygenase inhibition. Levels of 12/15-LOX are increased in both neurons and endothelial cells of the peri-infarct region after transient focal ischemia in mice, suggesting that 12/15-LOX may contribute to damage in both cell types. Although the exact mechanism of cell injury is still being investigated, in several cell types, 12/15-LOX contributes to degradation of mitochondria and other organelles.18–20 In endothelial cells, additional degradative pathways may be activated. In rat heart endothelial cells, the 12/15-LOX inhibitor baicalein has been reported to protect integrins, guarding against breakdown of intercellular connections.21 Similarly, in our in vivo model of transient focal ischemia, we find that baicalein treatment preserves claudin-5, a component of the tight junction connecting endothelial cells to one another and thus forming part of the BBB. To investigate if BBB leakage is similarly reduced, we detected extravascular IgG by immunohistochemistry. There was a significant reduction of IgG staining in the baicalein-treated brains, suggesting an improved preservation of the BBB. One of the possible consequences of reduced BBB leakage might be a reduction in vasogenic edema. We therefore compared the water content of ischemic brain hemispheres and detected a reduction of edema formation by baicalein treatment. Baicalein is likely to act through inhibition of 12/15-LOX, because mice in which the ALOX15 gene encoding 12/15-LOX has been knocked out show both a similar reduction in IgG extravasation and in edema formation. One caveat here concerns the possibility that reduced edema in either baicalein-treated mice or 12/15-LOX knockout mice might simply reflect the smaller infarct size; however, enhanced preservation of the tight junction protein claudin-5 in baicalein-treated mice suggests that edema reduction may be a specific effect of lipoxygenase inhibition. Moreover, IgG extravasation indicative of BBB disruption does not necessarily correlate with infarct size reduction,8 and it too is significantly reduced in both baicalein-treated and ALOX15 knockout mice. Future studies to determine the time course of edema formation in the presence or absence of baicalein may contribute to answering this important question.
It is remarkable that not all activities of 12/15-LOX are detrimental to the brain. 12/15-LOX also can generate resolvins and protectins from the longer chain polyunsaturated fatty acids eicosapentaenoic acid and docosahexaenoic acid, respectively. These are important anti-inflammatory compounds that may serve to limit brain damage.22–25 It will be interesting to see the long-term outcome effects of 12/15-LOX inhibition. In the transient focal ischemia model used here, however, the damaging effects of 12/15-LOX on neurons and endothelial cells appear to outweigh possible benefits through anti-inflammatory effects.
Interestingly, several compounds known to reduce edema in animal models of ischemia are also known lipoxygenase inhibitors: the enzyme glutathione peroxidase when overexpressed in mice26,27 as well as small molecule compounds like edaravone,28 melatonin,29,30 curcumin,31 AA-861,32,33 and green tea polyphenols like epigallocatechin gallate.34,35 Furthermore, some compounds may protect indirectly, in part by reducing lipid peroxidation, which prevents the activation of 12/15-LOX.36 Examples of this are the lazaroids37 and the newly investigated compound AS101.38 Although clearly varied mechanisms are targeted by these treatments, 12/15-LOX inhibition may contribute to the reduction of edema in these models as well.
Overall, the results from our cell culture and animal experiments suggest that 12/15-LOX may contribute to endothelial injury and damage to the BBB. Consistent with this detrimental role of endothelial 12/15-LOX, lipoxygenase inhibition with baicalein in vivo or gene knockout led to reduced formation of edema. Combined with our previously published results, these findings suggest a multimodal form of protection against ischemic damage through inhibition of 12/15-LOX.
Acknowledgments
Sources of Funding
Support through grants from the National Institutes of Health (R01NS049430 to KvL, R01NS53560 and P01NS555104 to EHL) and a Scientist Development Grant from the American Heart Association (to KvL) is gratefully acknowledged.
Footnotes
Disclosures
None.
References
- 1.Hacke W, Schwab S, Horn M, Spranger M, De Georgia M, von Kummer R. ‘Malignant’ middle cerebral artery territory infarction: clinical course and prognostic signs. Arch Neurol. 1996;53:309–315. doi: 10.1001/archneur.1996.00550040037012. [DOI] [PubMed] [Google Scholar]
- 2.Heo JH, Han SW, Lee SK. Free radicals as triggers of brain edema formation after stroke. Free Radic Biol Med. 2005;39:51–70. doi: 10.1016/j.freeradbiomed.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 3.van Leyen K, Kim HY, Lee SR, Jin G, Arai K, Lo EH. Baicalein and 12/15-lipoxygenase in the ischemic brain. Stroke. 2006;37:3014–3018. doi: 10.1161/01.STR.0000249004.25444.a5. [DOI] [PubMed] [Google Scholar]
- 4.Stins MF, Badger J, Sik Kim K. Bacterial invasion and transcytosis in transfected human brain microvascular endothelial cells. Microb Pathog. 2001;30:19–28. doi: 10.1006/mpat.2000.0406. [DOI] [PubMed] [Google Scholar]
- 5.Connolly ES, Jr, Winfree CJ, Stern DM, Solomon RA, Pinsky DJ. Procedural and strain-related variables significantly affect outcome in a murine model of focal cerebral ischemia. Neurosurgery. 1996;38:523–531. doi: 10.1097/00006123-199603000-00021. [DOI] [PubMed] [Google Scholar]
- 6.Willis CL, Leach L, Clarke GJ, Nolan CC, Ray DE. Reversible disruption of tight junction complexes in the rat blood–brain barrier, following transitory focal astrocyte loss. Glia. 2004;48:1–13. doi: 10.1002/glia.20049. [DOI] [PubMed] [Google Scholar]
- 7.Fukui S, Fazzina G, Amorini AM, Dunbar JG, Marmarou A. Differential effects of atrial natriuretic peptide on the brain water and sodium after experimental cortical contusion in the rat. J Cereb Blood Flow Metab. 2003;23:1212–1218. doi: 10.1097/01.WCB.0000088762.02615.30. [DOI] [PubMed] [Google Scholar]
- 8.Remmers M, Schmidt-Kastner R, Belayev L, Lin B, Busto R, Ginsberg MD. Protein extravasation and cellular uptake after high-dose human-albumin treatment of transient focal cerebral ischemia in rats. Brain Res. 1999;827:237–242. doi: 10.1016/s0006-8993(99)01304-9. [DOI] [PubMed] [Google Scholar]
- 9.Lo EH, Pan Y, Matsumoto K, Kowall NW. Blood–brain barrier disruption in experimental focal ischemia: comparison between in vivo MRI and immunocytochemistry. Magn Reson Imaging. 1994;12:403–411. doi: 10.1016/0730-725x(94)92533-x. [DOI] [PubMed] [Google Scholar]
- 10.Shimamura N, Matchett G, Yatsushige H, Calvert JW, Ohkuma H, Zhang J. Inhibition of integrin alphavbeta3 ameliorates focal cerebral ischemic damage in the rat middle cerebral artery occlusion model. Stroke. 2006;37:1902–1909. doi: 10.1161/01.STR.0000226991.27540.f2. [DOI] [PubMed] [Google Scholar]
- 11.Dohmen C, Bosche B, Graf R, Staub F, Kracht L, Sobesky J, Neveling M, Brinker G, Heiss WD. Prediction of malignant course in MCA infarction by PET and microdialysis. Stroke. 2003;34:2152–2158. doi: 10.1161/01.STR.0000083624.74929.32. [DOI] [PubMed] [Google Scholar]
- 12.Klatzo I. Evolution of brain edema concepts. Acta Neurochir Suppl (Wien) 1994;60:3–6. doi: 10.1007/978-3-7091-9334-1_1. [DOI] [PubMed] [Google Scholar]
- 13.Rosenberg GA. Ischemic brain edema. Prog Cardiovasc Dis. 1999;42:209–216. doi: 10.1016/s0033-0620(99)70003-4. [DOI] [PubMed] [Google Scholar]
- 14.Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after sub-arachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- 15.Lo EH, Moskowitz MA, Jacobs TP. Exciting, radical, suicidal: how brain cells die after stroke. Stroke. 2005;36:189–192. doi: 10.1161/01.STR.0000153069.96296.fd. [DOI] [PubMed] [Google Scholar]
- 16.Fagan SC, Hess DC, Hohnadel EJ, Pollock DM, Ergul A. Targets for vascular protection after acute ischemic stroke. Stroke. 2004;35:2220–2225. doi: 10.1161/01.STR.0000138023.60272.9e. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Maher P, Schubert D. A role for 12-lipoxygenase in nerve cell death caused by glutathione depletion. Neuron. 1997;19:453–463. doi: 10.1016/s0896-6273(00)80953-8. [DOI] [PubMed] [Google Scholar]
- 18.van Leyen K, Duvoisin RM, Engelhardt H, Wiedmann M. A function for lipoxygenase in programmed organelle degradation. Nature. 1998;395:392–395. doi: 10.1038/26500. [DOI] [PubMed] [Google Scholar]
- 19.Yokota S, Oda T, Fahimi HD. The role of 15-lipoxygenase in disruption of the peroxisomal membrane and in programmed degradation of peroxisomes in normal rat liver. J Histochem Cytochem. 2001;49:613–622. doi: 10.1177/002215540104900508. [DOI] [PubMed] [Google Scholar]
- 20.Grüllich C, Duvoisin RM, Wiedmann M, van Leyen K. Inhibition of 15-lipoxygenase leads to delayed organelle degradation in the reticulocyte. FEBS Lett. 2001;489:51–54. doi: 10.1016/s0014-5793(01)02080-4. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh YC, Hsieh SJ, Chang YS, Hsueh CM, Hsu SL. The lipoxygenase inhibitor, baicalein, modulates cell adhesion and migration by up-regulation of integrins and vinculin in rat heart endothelial cells. Br J Pharmacol. 2007;151:1235–1245. doi: 10.1038/sj.bjp.0707345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcheselli VL, Hong S, Lukiw WJ, Tian XH, Gronert K, Musto A, Hardy M, Gimenez JM, Chiang N, Serhan CN, Bazan NG. Novel docosanoids inhibit brain ischemia–reperfusion-mediated leukocyte infiltration and pro-inflammatory gene expression. J Biol Chem. 2003;278:43807–43817. doi: 10.1074/jbc.M305841200. [DOI] [PubMed] [Google Scholar]
- 23.Bazan NG, Marcheselli VL, Cole-Edwards K. Brain response to injury and neurodegeneration: endogenous neuroprotective signaling. Ann N Y Acad Sci. 2005;1053:137–147. doi: 10.1196/annals.1344.011. [DOI] [PubMed] [Google Scholar]
- 24.Schwab JM, Chiang N, Arita M, Serhan CN. Resolvin e1 and protectin d1 activate inflammation-resolution programmes. Nature. 2007;447:869–874. doi: 10.1038/nature05877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schwab JM, Serhan CN. Lipoxins and new lipid mediators in the resolution of inflammation. Curr Opin Pharmacol. 2006;6:414–420. doi: 10.1016/j.coph.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Weisbrot-Lefkowitz M, Reuhl K, Perry B, Chan PH, Inouye M, Mirochnitchenko O. Overexpression of human glutathione peroxidase protects transgenic mice against focal cerebral ischemia/reperfusion damage. Brain Res Mol Brain Res. 1998;53:333–338. doi: 10.1016/s0169-328x(97)00313-6. [DOI] [PubMed] [Google Scholar]
- 27.Huang HS, Chen CJ, Suzuki H, Yamamoto S, Chang WC. Inhibitory effect of phospholipid hydroperoxide glutathione peroxidase on the activity of lipoxygenases and cyclooxygenases. Prostaglandins Other Lipid Mediat. 1999;58:65–75. doi: 10.1016/s0090-6980(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida H, Yanai H, Namiki Y, Fukatsu-Sasaki K, Furutani N, Tada N. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 2006;12:9–20. doi: 10.1111/j.1527-3458.2006.00009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reiter RJ, Tan DX, Leon J, Kilic U, Kilic E. When melatonin gets on your nerves: its beneficial actions in experimental models of stroke. Exp Biol Med (Maywood) 2005;230:104–117. doi: 10.1177/153537020523000205. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Akbar M, Kim HY. Melatonin: an endogenous negative modulator of 12-lipoxygenation in the rat pineal gland. Biochem J. 1999;344:487–493. [PMC free article] [PubMed] [Google Scholar]
- 31.Thiyagarajan M, Sharma SS. Neuroprotective effect of curcumin in middle cerebral artery occlusion induced focal cerebral ischemia in rats. Life Sci. 2004;74:969–985. doi: 10.1016/j.lfs.2003.06.042. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe T, Egawa M. Effects of an antistroke agent MCL-186 on cerebral arachidonate cascade. J Pharmacol Exp Ther. 1994;271:1624–1629. [PubMed] [Google Scholar]
- 33.Baskaya MK, Hu Y, Donaldson D, Maley M, Rao AM, Prasad MR, Dempsey RJ. Protective effect of the 5-lipoxygenase inhibitor aa-861 on cerebral edema after transient ischemia. J Neurosurg. 1996;85:112–116. doi: 10.3171/jns.1996.85.1.0112. [DOI] [PubMed] [Google Scholar]
- 34.Lee SY, Kim CY, Lee JJ, Jung JG, Lee SR. Effects of delayed administration of (−)-epigallocatechin gallate, a green tea polyphenol on the changes in polyamine levels and neuronal damage after transient forebrain ischemia in gerbils. Brain Res Bull. 2003;61:399–406. doi: 10.1016/s0361-9230(03)00139-4. [DOI] [PubMed] [Google Scholar]
- 35.Schewe T, Sadik C, Klotz LO, Yoshimoto T, Kuhn H, Sies H. Polyphenols of cocoa: inhibition of mammalian 15-lipoxygenase. Biol Chem. 2001;382:1687–1696. doi: 10.1515/BC.2001.204. [DOI] [PubMed] [Google Scholar]
- 36.Kühn H, Walther M, Kuban RJ. Mammalian arachidonate 15-lipoxygenases structure, function, and biological implications. Prostaglandins Other Lipid Mediat. 2002;68–69:263–290. doi: 10.1016/s0090-6980(02)00035-7. [DOI] [PubMed] [Google Scholar]
- 37.Hall ED. Inhibition of lipid peroxidation in central nervous system trauma and ischemia. J Neurol Sci. 1995;134 suppl:79–83. doi: 10.1016/0022-510x(95)00211-j. [DOI] [PubMed] [Google Scholar]
- 38.Okun E, Arumugam TV, Tang SC, Gleichmann M, Albeck M, Sredni B, Mattson MP. The organotellurium compound ammonium trichloro(dioxoethylene-0,0′) tellurate enhances neuronal survival and improves functional outcome in an ischemic stroke model in mice. J Neurochem. 2007;102:1232–1241. doi: 10.1111/j.1471-4159.2007.04615.x. [DOI] [PubMed] [Google Scholar]