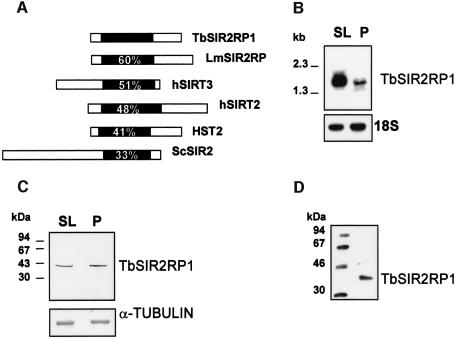
Fig. 1. Characterization of TbSIR2RP1. (A) Sequence comparison between TbSIR2RP1 and homologous proteins. The position of the conserved core domain is shown in black, and numbers represent the percentage amino acid identity with this region of TbSIR2RP1. The SIR2-like proteins compared are: LmSIR2RP from L.major (Yahiaoui et al., 1996), hSIRT2 and hSIRT3 from man (Frye, 1999) and HST2 and SIR2 from S.cerevisiae (Brachmann et al., 1995). (B) Northern blot analysis of TbSIR2RP1 mRNA. A 10 µg aliquot of poly(A)+ RNA from T.brucei AnTat 1.1 bloodstream long slender (SL) and procyclic forms (P) was separated on a formaldehyde agarose gel, transferred to nitrocellulose and probed with 32P-labelled TbSIR2RP1 cDNA. The filter was subsequently rehybridized with a ribosomal 18S probe, as control for RNA loading. (C) Immunoblot analysis of TbSIR2RP1. Total bloodstream slender (SL) and procyclic (P) cell lysates in SDS–PAGE sample buffer were examined by western blot analysis with affinity- purified anti-TbSIR2RP1. A control analysis was performed on a similar filter with an anti-α-tubulin monoclonal antibody. (D) Immunoprecipitation of in vitro translated TbSIR2RP1 with affinity-purified anti-TbSIR2RP1 antibodies.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.