Abstract
Decreased kidney function, determined using a serum creatinine–based estimation of GFR, is associated with a higher risk for mortality from cardiovascular disease. Equations incorporating cystatin C improve the estimation of GFR, but whether this improves the prediction of risk for mortality is unknown. We measured cystatin C on 6942 adult participants in the Third National Health and Nutrition Examination Survey Linked Mortality File, including all participants who had high serum creatinine (>1.2 mg/dl for men; >1.0 mg/dl for women) or were older than 60 yr and 25% random sample of participants who were younger than 60 yr. We estimated GFR using equations that included standardized serum creatinine, cystatin C, or both. Participant data were linked to the National Death Index. A total of 1573 (22.7%) deaths (713 deaths from cardiovascular disease) occurred during a median of 8 yr. Lower estimated GFR based on cystatin C was strongly associated with higher risk for overall and cardiovascular mortality across the range of normal to moderately decreased estimated GFR. Creatinine-based estimates of GFR resulted in weaker associations, with the association between estimated GFR and all-cause mortality reversed at higher levels of estimated GFR. An equation using both creatinine and cystatin C (in addition to age, race, and gender) resulted in weaker associations than equations using only cystatin C (with or without age, race, and gender). In conclusion, despite better performance in terms of estimating GFR, equations based on both cystatin C and creatinine do not predict mortality as well as equations based on cystatin C alone.
Moderately decreased kidney function, as estimated from equations based on serum creatinine, is associated with an elevated risk for mortality, both in individuals with existing cardiovascular disease (CVD) and in the general population.1–3 Serum levels of creatinine, however, are affected by other factors in addition to GFR, most importantly, variations in creatinine generation as a result of differences in muscle mass and turnover.4 Muscle wasting as a result of chronic illness is associated with lower creatinine generation, leading to an overestimation of GFR in such individuals. Because these same individuals are at an elevated risk for mortality, this systematic bias would result in an underestimation of the association between decreased GFR and mortality risk. This may be a particular problem in the elderly because of their higher prevalence of chronic illness and higher risk for mortality. In addition, currently available GFR estimates based on serum creatinine are less accurate at higher levels of kidney function, probably reflecting a greater proportional contribution of creatinine generation to variation in the serum creatinine level than at lower levels of kidney function.5
Cystatin C is a marker of kidney function that is less sensitive to differences in muscle mass than is serum creatinine.4 Cystatin C predicted total and cardiovascular mortality risk more strongly than creatinine-based estimates of GFR in prospective studies of older adults.6,7 Data on younger individuals are lacking. Recent data allow GFR estimation from serum cystatin C and showed that equations using both serum creatinine and cystatin C, in addition to age, race, and gender, are more closely correlated with directly measured GFR among individuals with chronic kidney disease (CKD) than equations based on either marker alone.8 The associations with mortality risk of eGFR based on serum creatinine, cystatin C, or the combination of the two, however, has not been studied. As the use of cystatin C as a marker of cardiovascular risk increases, it is critical to understand how one should combine information on serum creatinine with cystatin C for risk prediction. We analyzed up to 13 yr of mortality follow-up on 6942 participants in the Third National Health and Nutrition Examination Survey (NHANES III) to assess the association of eGFR based on equations using creatinine, cystatin C, or both markers with the risk for all-cause and cardiovascular mortality in a representative sample of US adults.
Results
Lower eGFR based on serum creatinine (eGFRcreat) was associated with older age, male gender, non-Hispanic white race/ethnicity, diabetes, prevalent coronary heart disease, hypertension, use of antihypertensive medication, lower prevalence of smoking, higher body mass index, higher LDL and lower HDL cholesterol, higher triglycerides, and higher C-reactive protein (CRP; Table 1). Serum cystatin C was strongly correlated with serum creatinine (r = 0.67; P < 0.001). eGFRcreat was similarly associated with eGFR using cystatin C alone (r = 0.69; P < 0.001) and eGFR estimated by cystatin C, age, race, and gender (eGFRcys; r = 0.71; P < 0.001). Table 2 shows the proportion of individuals in each eGFR category by eGFRcreat and each cystatin C equation. Agreement between eGFRcreat and cystatin C alone was 56.2% (κ = 0.35), and agreement between eGFRcreat and eGFRcys was 55.5% (κ = 0.36). Among individuals with an eGFRcreat between 30 and 119 ml/min per 1.73 m2, 15.9% were classified into a higher eGFR category by eGFRcys than by eGFRcreat, and 25.8% were classified into a lower eGFR category.
Table 1.
Participant characteristics, by age and eGFR: NHANES III
| Characteristic | eGFR Based on Serum Creatinine, Age, Race, and Gender (ml/min per 1.73 m2) |
||||
|---|---|---|---|---|---|
| ≥120 | 90 to 119 | 60 to 89 | 30 to 59 | 15 to 29 | |
| n | 720 | 2081 | 3232 | 868 | 41 |
| Creatinine (mg/dl) | 0.58 | 0.73 | 0.87 | 1.23 | 2.49 |
| eGFR based on serum creatinine, age, race, and gender (ml/min per 1.73 m2) | 138.4 | 102.9 | 76.4 | 49.8 | 23.6 |
| Cystatin C (mg/L) | 0.76 | 0.84 | 1.02 | 1.49 | 2.91 |
| eGFR based on cystatin C (ml/min per 1.73 m2) | 111.4 | 98.3 | 78.6 | 52.4 | 24.3 |
| eGFR based on cystatin C, age, race, and gender (ml/min per 1.73 m2) | 113.8 | 97.7 | 74.2 | 48.3 | 22.5 |
| Age (yr) | 35.4 | 46.2 | 63.9 | 73.4 | 73.9 |
| Female (%) | 61.7 | 49.6 | 51.3 | 56.3 | 61.0 |
| Race (%) | |||||
| non-Hispanic white | 16.3 | 35.0 | 57.3 | 68.8 | 51.2 |
| non-Hispanic black | 41.0 | 31.0 | 19.2 | 16.8 | 29.3 |
| Mexican American | 39.7 | 29.8 | 19.6 | 10.8 | 17.1 |
| other | 3.1 | 4.1 | 3.9 | 3.6 | 2.4 |
| Diabetes (%) | 6.1 | 6.8 | 10.5 | 17.5 | 41.5 |
| Prevalent CVD (%) | 4.3 | 8.5 | 18.7 | 39.5 | 48.8 |
| Hypertension category (%) | |||||
| optimal | 56.3 | 41.2 | 21.0 | 12.1 | 7.3 |
| prehypertension | 30.0 | 37.5 | 38.6 | 31.1 | 24.4 |
| stage 1 hypertension | 10.3 | 14.8 | 27.0 | 34.1 | 39.0 |
| stage 2 hypertension | 3.5 | 6.4 | 13.3 | 22.7 | 29.3 |
| Antihypertensive medication (%) | 17.1 | 28.9 | 54.5 | 82.1 | 97.6 |
| Current smoking (%) | 29.3 | 29.9 | 17.1 | 12.1 | 12.2 |
| Body mass index (kg/m2) | 26.6 | 26.8 | 27.4 | 27.3 | 28.2 |
| LDL cholesterol (mg/dl) | 112.2 | 121.6 | 135.2 | 140.2 | 140.8 |
| HDL cholesterol (mg/dl) | 52.5 | 51.1 | 51.1 | 49.9 | 50.8 |
| Triglycerides (mg/dl) | 132.4 | 132.6 | 154.7 | 180.5 | 232.4 |
| CRP (mg/dl; %) | |||||
| <0.22 | 63.9 | 66.8 | 62.2 | 54.0 | 46.3 |
| 0.22 to 0.99 | 25.4 | 23.2 | 28.2 | 31.0 | 36.6 |
| ≥1.00 | 10.7 | 10.1 | 9.6 | 15.0 | 17.1 |
Table 2.
Proportion of participants in each category of eGFR: NHANES III
| Parameter | eGFR Based on Serum Creatinine, Age, Race, and Gender (ml/min per 1.73 m2) |
|||||
|---|---|---|---|---|---|---|
| ≥120 | 90 to 119 | 60 to 89 | 30 to 59 | 15 to 29 | Overall (Row %) | |
| Overall (column %) | 720 (10.4) | 2081 (30.0) | 3232 (46.6) | 868 (12.5) | 41 (0.6) | 6942 |
| eGFR based on cystatin C (ml/min per 1.73 m2) | ||||||
| ≥120 | 226 (31.4) | 244 (11.7) | 57 (1.8) | 2 (0.2) | 0 (0.0) | 539 (7.6) |
| 90 to 119 | 387 (53.8) | 1136 (54.6) | 741 (22.9) | 21 (2.4) | 0 (0.0) | 2285 (32.9) |
| 60 to 89 | 99 (13.8) | 661 (31.8) | 1968 (60.9) | 232 (26.7) | 1 (2.4) | 2961 (42.7) |
| 30 to 59 | 8 (1.1) | 40 (1.9) | 460 (14.2) | 539 (62.1) | 6 (14.6) | 1053 (15.2) |
| <30 | 0 (0.0) | 0 (0.0) | 6 (0.2) | 74 (8.5) | 34 (82.9) | 114 (1.6) |
| eGFR based on cystatin C, age, race, and gender (ml/min per 1.73 m2) | ||||||
| ≥120 | 271 (37.6) | 279 (13.4) | 54 (1.7) | 2 (0.2) | 0 (0.0) | 606 (8.7) |
| 90 to 119 | 341 (47.4) | 1057 (50.8) | 531 (16.4) | 13 (1.5) | 0 (0.0) | 1942 (28.0) |
| 60 to 89 | 99 (13.8) | 674 (32.4) | 1872 (57.9) | 163 (18.8) | 1 (2.4) | 2809 (40.5) |
| 30 to 59 | 9 (1.3) | 71 (3.4) | 769 (23.8) | 616 (71.0) | 6 (14.6) | 1471 (21.2) |
| <30 | 0 (0.0) | 0 (0.0) | 6 (0.2) | 74 (8.5) | 34 (82.9) | 114 (1.6) |
| eGFR based on serum creatinine, cystatin C, age, race, and gender (ml/min per 1.73 m2) | ||||||
| ≥120 | 549 (76.3) | 265 (12.7) | 6 (0.2) | 0 (0.0) | 0 (0.0) | 820 (11.8) |
| 90 to 119 | 169 (23.5) | 1517 (72.9) | 474 (14.7) | 0 (0.0) | 0 (0.0) | 2160 (31.1) |
| 60 to 89 | 2 (0.3) | 299 (14.4) | 2474 (76.55) | 110 (12.7) | 0 (0.0) | 2885 (41.6) |
| 30 to 59 | 0 (0.0) | 0 (0.0) | 272 (8.42) | 684 (78.8) | 5 (12.2) | 961 (13.8) |
| <30 | 0 (0.0) | 0 (0.0) | 6 (0.2) | 74 (8.5) | 36 (87.8) | 116 (1.7) |
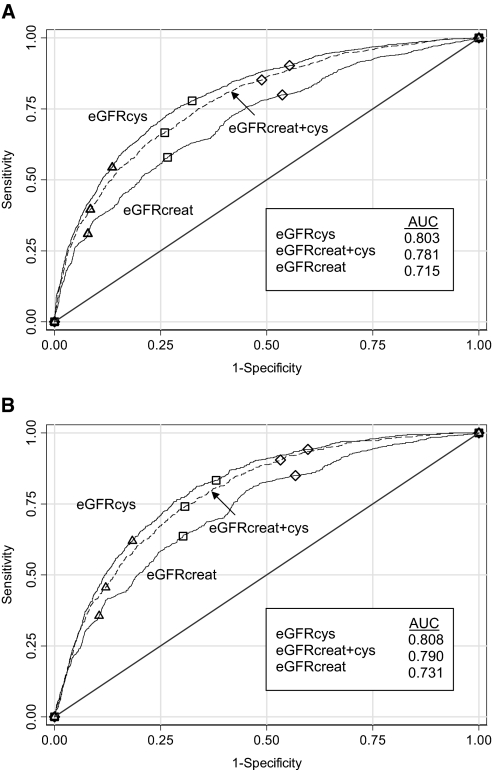
A total of 1573 (22.7%) participants died during a median follow-up of 8.0 yr. A total of 713 (10.3%) participants died of CVD. Receiver operating characteristic curves (Figure 1) show that eGFRcys was most predictive of all-cause mortality, with an area under the curve (AUC) of 0.803, compared with an AUC of 0.715 for eGFRcreat and 0.781 for eGFRcreat+cys (both P < 0.001). The curve for eGFR based on cystatin C alone (not shown) was very similar to that for eGFRcys (AUC = 0.792). Results were similar for cardiovascular mortality (Figure 1B).
Figure 1.
(A and B) Receiver operating characteristics for all-cause (A) and cardiovascular (B) mortality for three GFR estimating equations. ▵, 60 ml/min per 1.73 m2; □, 75 ml/min per 1.73 m2; ♢, 90 ml/min per 1.73 m2. All P < 0.001 versus eGFRcreat.
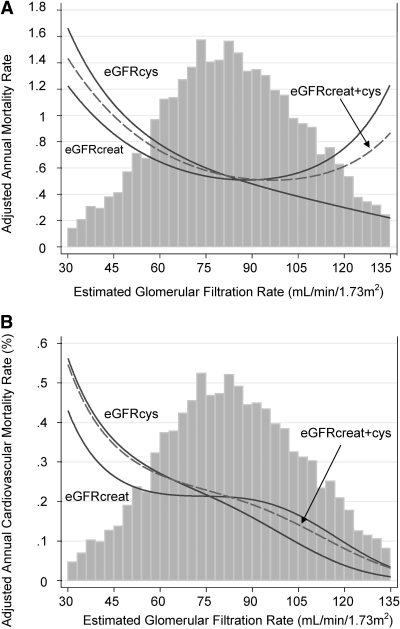
Figure 2 shows the relationship between eGFR, analyzed as a continuous variable, and all-cause (Figure 2A) and cardiovascular (Figure 2B) mortality. The relationship of decreasing eGFRcys with higher risk was monotonic, whereas the relationship with eGFRcreat was U-shaped. The relationship between eGFRcreat+cys was between the two other curves and exhibited a less pronounced U shape. The relationship with eGFR based on cystatin C alone (data not shown) was very similar to that of eGFRcys. All three curves were monotonic for cardiovascular mortality, with the steepest relationship again being with eGFRcys and the least steep with eGFRcreat (Figure 2B).
Figure 2.
(A and B) Adjusted annual rate, by eGFR of all-cause mortality (A) and cardiovascular mortality (B). Incidence rates were adjusted to the incidence rate of a white woman with the lowest risk category for categorical covariates (smoking status, diabetes status, previous CVD, CRP category, and BP category) and the overall mean values of continuous covariates (age, body mass index, LDL and HDL cholesterol, and log triglycerides). Vertical bars represent histogram of the mean of all three eGFRs.
In fully adjusted analyses, decreased eGFRcreat had the weakest association with mortality (Table 3). The cystatin C–based equation including age, race, and gender was only slightly more strongly associated than the equation based on cystatin C alone. The equation based on both markers (eGFRcreat+cys) was less strongly associated with mortality than either cystatin C–based equation. Individuals with an elevated eGFRcreat (≥120 ml/min per 1.73 m2) were at 36% higher risk compared with those with a normal eGFRcreat (90 to 119 ml/min per 1.73 m2). No increased risk associated with elevated eGFR, compared with normal eGFR, was observed for either cystatin C–based estimate. A smaller increase (31%) was observed for the combined equation (eGFRcreat+cys). These patterns were similar for cardiovascular mortality, although the associations were stronger for all estimates of GFR and no increased risk at elevated eGFRcreat was observed.
Table 3.
Adjusted incidence rate ratio (95% confidence interval) of cardiovascular and all-cause mortality, by category of eGFR: NHANES IIIa
| Parameter | eGFR (ml/min per 1.73 m2) |
||||||
|---|---|---|---|---|---|---|---|
| ≥120 | 90 to 119 | 75 to 89 | 60 to 74 | 45 to 59 | 30 to 44 | 15 to 29 | |
| All-cause mortality | |||||||
| eGFR based on: | |||||||
| creatinine, age, race, and gender | 1.36 (1.01 to 1.82) | 1.00 (Ref) | 0.92 (0.78 to 1.08) | 0.98 (0.83 to 1.16) | 1.31 (1.09 to 1.56) | 1.80 (1.46 to 2.24) | 2.43 (1.67 to 3.53) |
| cystatin C | 1.06 (0.67 to 1.68) | 1.00 (Ref) | 1.07 (0.88 to 1.30) | 1.53 (1.27 to 1.84) | 1.97 (1.62 to 2.41) | 2.76 (2.21 to 3.45) | 3.97 (2.93 to 5.39) |
| cystatin C, age, race, and gender | 0.90 (0.54 to 1.50) | 1.00 (Ref) | 0.89 (0.71 to 1.11) | 1.21 (0.98 to 1.51) | 1.53 (1.22 to 1.92) | 2.45 (1.93 to 3.13) | 3.46 (2.56 to 4.68) |
| creatinine, cystatin C, age, race, and gender | 1.16 (0.78 to 1.73) | 1.00 (Ref) | 0.96 (0.80 to 1.15) | 1.13 (0.94 to 1.36) | 1.54 (1.26 to 1.87) | 2.00 (1.61 to 2.50) | 2.86 (2.03 to 4.03) |
| Cardiovascular mortality | |||||||
| eGFR based on: | |||||||
| creatinine, age, race, and gender | 0.83 (0.44 to 1.56) | 1.00 (Ref) | 1.01 (0.78 to 1.31) | 1.06 (082 to 1.37) | 1.42 (1.08 to 1.87) | 2.19 (1.61 to 2.99) | 2.54 (1.46 to 4.41) |
| cystatin C | 0.95 (0.38 to 2.38) | 1.00 (Ref) | 1.21 (0.86 to 1.69) | 1.83 (1.34 to 2.51) | 2.73 (1.97 to 3.79) | 3.56 (2.49 to 5.08) | 5.47 (3.47 to 8.63) |
| cystatin C, age, race, and gender | 0.93 (0.33 to 2.63) | 1.00 (Ref) | 1.13 (0.76 to 1.69) | 1.49 (1.02 to 2.19) | 2.13 (1.44 to 3.14) | 3.56 (2.37 to 5.36) | 4.40 (2.69 to 7.18) |
| creatinine, cystatin C, age, race, and gender | 1.18 (0.53 to 2.61) | 1.00 (Ref) | 1.16 (0.84 to 1.59) | 1.48 (1.09 to 2.01) | 2.01 (1.46 to 2.77) | 2.83 (2.00 to 4.00) | 3.94 (2.39 to 6.49) |
aAdjusted for age, gender, race/ethnicity, previous CVD, BP category, use of antihypertensive medication, diabetes status, smoking status, body mass index, LDL and HDL cholesterol, log triglycerides, and CRP category.
Table 4 shows the adjusted incidence rate ratio for each eGFRcreat category and eGFRcys category, compared with an eGFRcreat and eGFRcys of 90 to 119 ml/min per 1.73 m2. An eGFRcys <60 ml/min per 1.73 m2 was associated with a statistically significantly elevated risk for all-cause mortality overall and at every eGFRcreat category. Lower eGFRcys category was significantly associated (P < 0.04) with higher all-cause and cardiovascular mortality risk for all but the lowest (15 to 29 ml/min per 1.73 m2) eGFRcreat categories. The only significant associations of eGFRcreat with risk were in the eGFRcys category of 60 to 89 ml/min per 1.73 m2, in which lower eGFRcreat category was associated with lower risk for all-cause (P = 0.002) and cardiovascular (P = 0.005) mortality.
Table 4.
Adjusted incidence rate ratio (95% confidence interval) of all-cause and cardiovascular mortality, by category of eGFR: NHANES IIIa
| Parameter | eGFR Based on Creatinine, Age, Race, and Gender (ml/min per 1.73 m2) |
|||||
|---|---|---|---|---|---|---|
| ≥120 | 90 to 119 | 60 to 89 | 30 to 59 | 15 to 29 | Overall | |
| All-cause mortality: eGFR based on cystatin C, age, race, and gender (ml/min per 1.73 m2) | ||||||
| ≥120 | 0.48 (0.15 to 1.54) | 1.38 (0.76 to 2.50) | 0.36 (0.05 to 2.62) | b | b | 0.93 (0.56 to 1.56) |
| 90 to 119 | 1.32 (0.83 to 2.11) | 1.00 (Ref) | 0.76 (0.51 to 1.13) | b | b | 1.00 (Ref) |
| 60 to 89 | 1.89 (1.19 to 3.00) | 1.05 (0.78 to 1.41) | 0.94 (0.72 to 1.22) | 1.01 (0.68 to 1.50) | b | 1.03 (0.84 to 1.26) |
| 30 to 59 | b | 1.73 (1.14 to 2.61) | 1.46 (1.10 to 1.94) | 1.76 (1.32 to 2.35) | b | 1.68 (1.35 to 2.09) |
| <30 | b | b | 2.38 (1.01 to 53.61) | 3.33 (2.30 to 4.81) | 2.90 (1.81 to 4.63) | 3.25 (2.42 to 4.37) |
| Overall | 1.36 (1.02 to 1.83) | 1.00 (Ref) | 0.94 (0.81 to 1.09) | 1.42 (1.20 to 1.68) | 2.39 (1.64 to 3.48) | |
| Cardiovascular mortality: eGFR based on cystatin C, age, race, and gender (ml/min per 1.73 m2) | ||||||
| ≥120 | c | 1.61 (0.55 to 4.70) | c | b | b | 0.97 (0.34 to 2.73) |
| 90 to 119 | 0.60 (0.18 to 2.02) | 1.00 (Ref) | 0.75 (0.37 to 1.52) | b | b | 1.00 (Ref) |
| 60 to 89 | 1.87 (0.82 to 4.26) | 1.17 (0.70 to 1.97) | 1.12 (0.70 to 1.79) | 0.92 (0.46 to 1.84) | b | 1.29 (0.90 to 1.86) |
| 30 to 59 | b | 2.13 (1.09 to 4.14) | 1.83 (1.12 to 3.00) | 2.40 (1.47 to 3.93) | b | 2.39 (1.63 to 3.50) |
| <30 | b | b | b | 3.61 (1.96 to 6.63) | 3.74 (1.82 to 7.67) | 4.15 (2.56 to 6.71) |
| Overall | 0.84 (0.45 to 1.57) | 1.00 (Ref) | 1.03 (0.82 to 1.30) | 1.61 (1.25 to 2.08) | 2.50 (1.44 to 4.34) | |
aAdjusted for age, gender, race/ethnicity, previous CVD, BP category, use of antihypertensive medication, diabetes status, smoking status, body mass index, LDL and HDL cholesterol, log triglycerides, and CRP category.
bn < 30.
cNo deaths.
Below an eGFR of 60 ml/min per 1.73 m2, each 10-ml/min per 1.73 m2 lower eGFR was associated with a 25 to 35% higher adjusted incidence of all-cause mortality for all estimating equations (Table 5). This was slightly higher for women (36%) than men (14%; P = 0.05 for interaction) for eGFRcreat, but no other differences were found for any estimating equation. At an eGFR of 60 to 89 ml/min per 1.73 m2, eGFRcreat was not associated with any change in risk, whereas each 10-ml/min per 1.73 m2 lower GFR estimated by the cystatin C–based equations were associated with 22 to 48% higher risk for all gender, race, and age subgroups, with the exception of the equation using cystatin C alone in those who were younger than 65 yr, although this estimate is based on only 108 deaths. No difference was found between those age <65 and ≥65 yr using eGFR based on cystatin C, age, race, and gender.
Table 5.
Adjusted incidence rate ratio for all-cause mortality per 10-ml/min per 1.73 m2 lower eGFR: NHANES III
| Parameter | Overall | Female | Male | P (Interaction) |
|---|---|---|---|---|
| eGFR <60 ml/min per 1.73 m2, based on: | ||||
| creatinine, age, race, and gender | 1.25 (1.31 to 1.37) | 1.36 (1.19 to 1.56) | 1.14 (0.99 to 1.32) | 0.05 |
| cystatin C | 1.32 (1.23 to 1.42) | 1.31 (1.18 to 1.45) | 1.32 (1.19 to 1.46) | 0.77 |
| cystatin C, age, race, and gender | 1.35 (1.27 to 1.44) | 1.41 (1.29 to 1.54) | 1.29 (1.17 to 1.42) | 0.13 |
| creatinine, cystatin C, age, race, and gender | 1.31 (1.21 to 1.42) | 1.37 (1.23 to 1.52) | 1.23 (1.10 to 1.39) | 0.13 |
| eGFR 60 to 90 ml/min per 1.73 m2, based on: | ||||
| creatinine, age, race, and gender | 1.00 (0.91 to 1.09) | 0.98 (0.86 to 1.11) | 1.01 (0.89 to 1.14) | 0.54 |
| cystatin C | 1.23 (1.11 to 1.35) | 1.25 (1.08 to 1.45) | 1.22 (1.07 to 1.40) | 0.78 |
| cystatin C, age, race, and gender | 1.29 (1.16 to 1.44) | 1.24 (1.04 to 1.49) | 1.35 (1.18 to 1.56) | 0.80 |
| creatinine, cystatin C, age, race, and gender | 1.12 (1.02 to 1.23) | 1.13 (0.98 to 1.30) | 1.13 (0.99 to 1.28) | 0.99 |
| Parameter | Black | Nonblack | P (Interaction) | Age <65 | Age ≥65 | P (Interaction) |
|---|---|---|---|---|---|---|
| eGFR <60 ml/min per 1.73 m2, based on: | ||||||
| creatinine, age, race, and gender | 1.24 (1.00 to 1.53) | 1.24 (1.11 to 1.38) | 0.83 | 1.26 (0.88 to 1.81) | 1.25 (1.12 to 1.38) | 0.41 |
| cystatin C | 1.22 (1.02 to 1.46) | 1.32 (1.22 to 1.44) | 0.62 | 1.30 (0.98 to 1.71) | 1.32 (1.22 to 1.43) | 0.92 |
| cystatin C, age, race, and gender | 1.31 (1.10 to 1.56) | 1.34 (1.25 to 1.44) | 0.91 | 1.47 (1.12 to 1.93) | 1.34 (1.25 to 1.43) | 0.08 |
| creatinine, cystatin C, age, race, and gender | 1.28 (1.07 to 1.53) | 1.30 (1.19 to 1.42) | 0.62 | 1.43 (1.04 to 1.97) | 1.30 (1.20 to 1.41) | 0.22 |
| eGFR 60 to 90 ml/min per 1.73 m2, based on: | ||||||
| creatinine, age, race, and gender | 1.01 (0.79 to 1.28) | 0.99 (0.90 to 1.09) | 0.61 | 0.97 (0.76 to 1.25) | 1.00 (0.91 to 1.10) | 0.63 |
| cystatin C | 1.29 (1.04 to 1.60) | 1.22 (1.09 to 1.36) | 0.91 | 1.01 (0.80 to 1.29) | 1.27 (1.14 to 1.42) | 0.04 |
| cystatin C, age, race, and gender | 1.48 (1.19 to 1.84) | 1.23 (1.09 to 1.40) | 0.78 | 1.30 (1.04 to 1.64) | 1.29 (1.13 to 1.46) | 0.36 |
| creatinine, cystatin C, age, race, and gender | 1.11 (0.88 to 1.40) | 1.13 (1.01 to 1.25) | 0.31 | 1.21 (0.94 to 1.56) | 1.11 (1.00 to 1.23) | 0.80 |
Discussion
In this study of the general population of US adults, lower eGFR based on cystatin C was strongly associated with higher risk for all-cause and cardiovascular mortality across the range of eGFR, from normal to moderately decreased. Similar associations were observed across subgroups defined by age, race, or gender. Creatinine-based estimates of GFR resulted in weaker associations, with the association between eGFR and all-cause mortality reversed at higher levels of eGFR. An equation using both creatinine and cystatin C, in addition to age, race, and gender, resulted in weaker associations than equations using only cystatin C, with or without age, race, and gender.
These results are in general agreement with previous studies showing that higher cystatin C predicts cardiovascular and all-cause mortality in older adults. Among individuals aged ≥65 yr in the Cardiovascular Health Study, higher cystatin C was associated with higher risk for cardiovascular events and mortality across the range of values.7 In contrast, only the lowest quintile of serum creatinine–based eGFR was associated with increased risk. Cystatin C levels >1.0 mg/L also predicted cardiovascular events and mortality among participants with a creatinine-based eGFR >60 ml/min per 1.73 m2.9,10 The Health, Aging and Body Composition (Health ABC) study similarly found cystatin C to be more strongly associated than creatinine with risk for mortality in the elderly.6 The results of this study confirm these associations in the general population and extend the findings to younger individuals and to subgroups defined by race and gender.
In contrast to previous studies that used only cystatin C without adjustment for age, race, or gender, we also used the recently derived CKD-EPI equation to estimate GFR (eGFRcys). The CKD-EPI equation has less bias, relative to directly measured GFR, than cystatin C alone in older individuals, black individuals, and women.8 Despite the improved estimation of GFR, there was only slight improvement in the overall prediction of risk between GFR estimated with or without these factors. The only substantial difference found between risk associated with these two equations was that GFR estimated by the equation without age, race, and gender was associated with risk among older (age ≥65 yr) but not younger individuals with an eGFR of 60 to 89 ml/min per 1.73 m2, whereas the equation including age, race, and gender was similarly associated in both younger and older individuals. The estimate of risk associated with GFR estimated from cystatin C alone in younger participants, however, was based on only 108 deaths. This suggests that adjustment for age is important for younger individuals but less so among older individuals. The reasons for this seemingly differential cystatin C–GFR relationship in younger compared with older individuals are unknown. Using the CKD-EPI equation, a cystatin C value of 1.0 mg/L equates to an eGFR of 75, 68, 80, and 72 ml/min per 1.73 m2 in a 60-yr old white man, white woman, black man, and black woman, respectively.
The equation using both creatinine and cystatin C, in addition to age, race, and gender, further improves the accuracy of GFR estimation, compared with either cystatin C equation, among individuals with CKD.8 It remains to be seen whether the combined equation also results in more accurate GFR estimation in the general population, as studied here. Because decreased GFR estimates from creatinine and from cystatin C both are associated with greater mortality risk, one may expect that an equation using both markers would result in stronger associations than equations based on either single marker. We found, however, that this equation resulted in weaker associations than the equations based on cystatin C only. Once accounting for eGFRcys category, mortality risk decreased with lower eGFRcreat category. These data are consistent with the hypothesis that GFR estimates based on cystatin C are superior to creatinine-based estimates in defining individuals who have decreased kidney function and may be at increased risk for cardiovascular complications and mortality. Substantial evidence supports the hypothesis that GFR is inversely associated with mortality. Given this, we conclude that once GFR is estimated by cystatin C, any improvement in estimating GFR by including creatinine is outweighed by the association of higher creatinine with higher muscle mass and, concomitantly, lower risk. This was observed not only at elevated eGFR levels, where one may expect lower creatinine levels to indicate chronic illness and muscle wasting, but also among those with mildly decreased kidney function (eGFR 60 to 89 ml/min per 1.73 m2).
The observed U-shaped association between higher creatinine-based eGFR and mortality risk may be due to the overestimation of measured GFR in individuals with lower muscle mass and therefore lower creatinine, such as those with chronic illness or inflammation. This would have the effect of misclassifying these individuals as having normal or elevated kidney function, when in fact their true GFR is low. Because cystatin C is thought to be less influenced by muscle mass than is creatinine, this misclassification would not be expected with cystatin C.11 This increased risk at elevated eGFRcreat levels was observed for all-cause mortality but not cardiovascular mortality, suggesting that the conditions associated with decreased creatinine are related only to noncardiovascular causes of death.
In summary, we found that decreased GFR estimated by cystatin C was independently and continuously associated with all-cause and cardiovascular mortality in the general population of the United States. Despite better performance in terms of estimating GFR, using equations based on both cystatin C and creatinine performed less well for predicting mortality. It remains to be seen whether the more precise estimation of GFR with the combined equation will lead to better prediction of outcomes that may be more closely associated with GFR, such as CKD progression, kidney failure, and acute kidney injury.
Concise Methods
Study Sample
This study uses data on participants aged ≥18 yr in the NHANES III. Baseline data collection was conducted during 1988 through 1994 by the National Center for Health Statistics of the Centers for Disease Control and Prevention. Mortality follow-up was conducted by linkage to National Death Index (NDI) records. The NHANES III used a complex, multistage clustering sampling design and provides cross-sectional, nationally representative data on the health and nutritional status of the civilian, noninstitutionalized US population.12,13 Non-Hispanic black, Mexican American, and elderly individuals were deliberately oversampled, allowing calculation of more precise estimates of the distribution of variables in these groups. The NDI records provided data on the date of death and underlying and multiple causes of death.
Cystatin C was measured in a subsample of 7596 NHANES III participants with nonmissing serum creatinine data. Included in this study sample are all participants who were older than 60 yr, a 25% random sample of all participants who were 12 to 59 yr, and all participants with high serum creatinine (>1.2 mg/dl in men; >1.0 mg/dl in women).14
Measurements
Standardized questionnaires were administered in the home, followed by a physical examination and serum collection at a mobile examination center, as described previously.12 Race was self-reported and categorized as non-Hispanic white, non-Hispanic black, Mexican American, or other. A participant was considered to have diabetes when he or she reported ever having been told by a doctor that he or she had diabetes or “sugar diabetes” at a time other than during pregnancy or he or she was taking insulin or a “diabetes pill” at the time of the questionnaire. Use of antihypertensive medication and previous CVD were defined by self-report. Previous CVD was considered positive when the participant reported ever having been told that he or she had a heart attack, heart failure, or stroke or had undergone a procedure for any of these conditions.
Serum creatinine was measured by the modified kinetic method of Jaffe using a Roche Hitachi 737 analyzer and calibrated to standardized creatinine.15 Cystatin C was measured by a particle-enhanced immunonephelometric assay (N Latex Cystatin C; Dade Behring, Somerville, NJ) with a nephelometer (BNII; Dade Behring).16,17 eGFRcreat was calculated by the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation, reexpressed for standardized creatinine: eGFRcreat = 175 × (serum creatinine in mg/dl)−1.154× (age in years)−0.203× (0.742 if female) × (1.21 if black).18 eGFRcys was calculated by the CKD-EPI equation: eGFR = 127.7 × (cystatin C in mg/L)−1.17× (age in years)−0.13× (0.91 if female) × (1.06 if black).8 In addition, GFR was estimated using cystatin C alone: eGFR = by 76.7 × (cystatin C in mg/L)−1.19. eGFRcreat+cys was calculated as follows: eGFRcreat+cys = 177.6 × (serum creatinine in mg/dl)−0.65× (cystatin C in mg/L)−0.57× (age in years)−0.20× (0.82 if female) × (1.11 if black). eGFR is reported in ml/min per 1.73 m2 body surface area. CRP was measured by latex-enhanced nephelometry (Dade Behring).
Linkage and Causes of Death
Linkage of NHANES III and NDI records through December 31, 2000, was performed by probabilistic matching, using up to 12 identifying data items.19 A selected sample of death certificates was reviewed manually to validate the process. The underlying cause of death was coded according to the International Statistical Classification of Diseases, Injuries, and Causes of Death, Ninth Revision (ICD-9) for deaths occurring between 1988 and 1998 and according to the International Statistical Classification of Diseases, Injuries, and Causes of Death, 10th Revision (ICD-10) for deaths occurring between 1999 and 2000.20,21 Underlying causes of death were grouped by National Center for Health Statistics for each coding system, and all deaths from 1988 through 1998 coded under ICD-9 guidelines were recoded into comparable groups on the basis of the ICD-10 underlying cause of death.22 For this analysis, cardiovascular disease mortality included deaths coded as due to hypertensive disease (I10 through I13), ischemic heart disease (I20 through I25), arrhythmia (I44 through I49), heart failure (I50), cerebrovascular disease (I60 through I69), or atherosclerosis or other diseases of the arteries (I70 through I78).
Statistical Analysis
eGFR was analyzed both as a continuous measure and divided into five categories (severely decreased: 15 to 29; moderately decreased: 30 to 59; mildly decreased: 60 to 89; normal: 90 to 119; and elevated: ≥120 ml/min per 1.73 m2).23 Participants with a creatinine-based eGFR <15 ml/min per 1.73 m2 (n = 18) were excluded from all analyses. Individuals with a physiologically implausibly high eGFR were assigned a maximum of 200 ml/min per 1.73 m2 (n = 16 using creatinine). Systolic (SBP) and diastolic (DBP) BP were collapsed into a joint categorical variable (normal: SBP <120 and DBP <80; prehypertension: SBP 120 to 139 or DBP 80 to 89; stage 1 hypertension: SBP 140 to 159 or DBP 90 to 99; stage 2 hypertension SBP ≥160 or DBP ≥100).24 CRP was categorized as undetectable by the assay (<0.22 mg/dl), minimal (0.22 to 0.99 mg/dl), or elevated (≥1.0 mg/dl). Triglyceride levels were log-transformed because of a skewed distribution. Individuals who were missing data on any variable of interest also were excluded (n = 184).
Continuous and categorical variables were compared between eGFR categories using t tests and χ2 tests, respectively. Linear or logistic regression, as appropriate, was used to evaluate trends across decreasing eGFR categories. Associations of eGFR with mortality were examined using multivariable Poisson regression models, which yielded similar results to Cox proportional hazards regression. Analyses were repeated after stratification by age (<65 or ≥65 yr), gender, and race/ethnicity. Third-order polynomial models were used to examine the shape of the association between eGFR and mortality. The adjusted incidence rates were calculated for a white female with the lowest risk category for categorical covariates (smoking status, diabetes status, previous CVD, CRP category, and BP category) and the overall mean values of continuous covariates (age, body mass index, LDL and HDL cholesterol, and log triglycerides). Receiver operating characteristic curves were drawn and the AUC was calculated for each equation. Results are presented separately for all-cause and cardiovascular mortality. Analyses were performed using Stata software (StataCorp, College Station, TX).25
Disclosures
None.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “More Evidence that Cystatin C Predicts Mortality Better than Creatinine,” on pages 2088–2090.
REFERENCES
- 1.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Astor BC, Sarnak MJ: Evidence for increased cardiovascular disease risk in patients with chronic kidney disease. Curr Opin Nephrol Hypertens 13: 73–81, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Stevens LA, Coresh J, Greene T, Levey AS: Assessing kidney function: Measured and estimated glomerular filtration rate. N Engl J Med 354: 2473–2483, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS: Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis 39: 920–929, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Shlipak MG, Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF: Cystatin C and mortality risk in the elderly: The health, aging, and body composition study. J Am Soc Nephrol 17: 254–261, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons SHLIPAK2005. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, III, Zhang YL, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: A pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51: 395–406, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shlipak MG, Fried LF, Crump C, Bleyer AJ, Manolio TA, Tracy RP, Furberg CD, Psaty BM: Cardiovascular disease risk status in elderly persons with renal insufficiency. Kidney Int 62: 997–1004, 2002 [DOI] [PubMed] [Google Scholar]
- 10.Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Filler G, Bokenkamp A, Hofmann W, Le Bricon T, Martinez-Bru C, Grubb A: Cystatin C as a marker of GFR: History, indications, and future research. Clin Biochem 38: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat 1 (32): 1–407, 1994 [PubMed] [Google Scholar]
- 13.Ezzati T, Wakesberg J, Chu A, Maurer K. Sample design: Third National Health and Nutrition Examination Survey, 1988–94. Vital Health Stat 2 (113): 1–35, 1992 [PubMed] [Google Scholar]
- 14.Kottgen A, Selvin E, Stevens LA, Levey AS, Van Lente F, Coresh J: Serum cystatin C in the United States: the Third National Health and Nutrition Examination Survey (NHANES III). Am J Kidney Dis 51: 385–394, 2008 [DOI] [PubMed] [Google Scholar]
- 15.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 16.Uhlmann EJ, Hock KG, Issitt C, Sneeringer MR, Cervelli DR, Gorman RT, Scott MG: Reference intervals for plasma cystatin C in healthy volunteers and renal patients, as measured by the Dade Behring BN II System, and correlation with creatinine. Clin Chem 47: 2031–2033, 2001 [PubMed] [Google Scholar]
- 17.Erlandsen EJ, Randers E, Kristensen JH: Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest 59: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F: Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Mason CA, Tu S: Data linkage using probabilistic decision rules: A primer. Birth Defects Res A Clin Mol Teratol 82: 812–821, 2008 [DOI] [PubMed] [Google Scholar]
- 20.International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) Washington, DC, US Department of Health and Human Services, Public Health Service, Health Care Financing Administration, 1991 [Google Scholar]
- 21.International Statistical Classification of Diseases and Related Health Problems, 10th Rev., Vol. 1 Geneva, World Health Organization; 1992 [Google Scholar]
- 22.Anderson RN, Minino AM, Hoyert DL, Rosenberg HM: Comparability of cause of death between ICD-9 and ICD-10: Preliminary estimates. Natl Vital Stat Rep 49: 1–32, 2001 [PubMed] [Google Scholar]
- 23.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification and stratification. Am J Kidney Dis 39[Suppl 1]: S1–S266, 2002 [PubMed] [Google Scholar]
- 24.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 report. JAMA 289: 2560–2572, 2003 [DOI] [PubMed] [Google Scholar]
- 25.StataCorp. Stata Statistical Software 1141 [Computer Program]. Release 8.1. College Station, TX, Stata Corp., 2003 [Google Scholar]