Abstract
Background
The aim of the current study was to determine the role of heparin-binding EGF-like growth factor (HB-EGF) as a mediator of gut barrier function after hemorrhagic shock and resuscitation (HS/R) in mice.
Methods
HS/R was induced in HB-EGF knockout (KO) and wild type (WT) mice. Intestinal histologic injury scores, intestinal epithelial cell apoptosis, and gut barrier function were determined. Statistical analyses were performed using linear mixed models with p<0.05 considered statistically significant.
Results
All mice subjected to HS/R had significantly increased intestinal histologic injury scores, apoptosis indices and intestinal permeability compared to mice subjected to sham surgery. Compared to WT mice, HB-EGF KO mice subjected to HS/R had significantly increased histologic injury (mean injury grade 4.5 ± 1 vs. 2.75 ± 0.5 at 3 h of resuscitation, p<0.05), increased apoptosis indices (mean apoptosis index 6.84 ± 1.95 vs. 3.24 ± 1.00 at 3 h of resuscitation, p<.05), and increased mucosal permeability (FD4 clearance 78 ± 18.91 vs. 47.75 ± 8.06 nl /min/ cm2 at 3 h of resuscitation, p<.05).
Conclusions
HB-EGF is essential for the preservation of gut barrier function after HS/R. These findings support the clinical use of HB-EGF in protection of the intestines from disease states associated with intestinal hypoperfusion injury.
Introduction
Ischemia/reperfusion (I/R) injury of the intestine is important in many clinically encountered critical situations including hemorrhagic shock and resuscitation (HS/R). The gut is particularly susceptible to I/R injury due to its higher critical oxygen requirement compared to other organs of the body. While the local effects of intestinal ischemia are a serious concern themselves, the systemic effects, manifested by the systemic inflammatory response syndrome and multiple organ dysfunction syndrome, can be devastating and often fatal.1, 2
Heparin-binding EGF-like growth factor (HB-EGF) was first identified as a 22-kDa glycoprotein in the conditioned medium of cultured human macrophages3, and later identified to be a member of the EGF family of growth factors.4 Like other EGF family members, HB-EGF binds to and activates the EGF receptor (EGFR/ HER1/ErbB-1). Additionally, HB-EGF activates ErbB-4 (HER4) and N-arginine dibasic convertase (NRDc), the latter representing a specific receptor for HB-EGF only.5 Mature HB-EGF has a strong affinity for cell-surface heparan-sulfate proteoglycans, which enhance its binding to EGFR and its bioactivity in some cell types.5
Previous studies in our laboratory revealed that endogenous HB-EGF was immediately upregulated in the intestine after I/R injury.6 Exogenous administration of HB-EGF decreases reactive oxygen species (ROS) production in the intestine7, and reduces intestinal I/R-induced inflammation.8 HB-EGF promotes intestinal epithelial cell (IEC) migration and restitution in order to heal superficial intestinal injuries9, and promotes angiogenesis in order to heal deep mucosal injuries.10,11 We have used three separate animal models of intestinal injury (I/R secondary to superior mesenteric artery occlusion6, HS/R12, and stress-induced necrotizing enterocolitis13) to show that HB-EGF can protect the intestines from each of these types of injury.
The current study was designed to examine the effects of loss of endogenous HB-EGF gene expression on gut barrier function using a clinically relevant model of HS/R.
Materials and Methods
Mouse model of hemorrhagic shock and resuscitation
HB-EGF(-/-) knockout (KO) mice on a C57BL/6J × 129 background and HB-EGF(+/+) wild type (WT) C57BL/6J × 129 mice were a kind gift from Dr David Lee (Chapel Hill, NC).14 In HB-EGF KO mice, HB-EGF exons 1 and 2 were replaced with PGK-Neo, thus deleting the signal peptide and propeptide domains. The desired targeting events were verified by Southern blots of genomic DNA and exon-specific polymerase chain reaction, with Northern blots confirming the absence of the respective transcripts.14 All animal procedures were approved by the Institutional Animal Care and Use Committee of the Children's Research Institute (Protocol 00903AR). Two- to three-month-old male HB-EGF KO or WT mice weighing 25 to 30g were fasted for 16 to 18 h with access to water only. Under inhalation anesthesia using 2% isoflurane, the right and left femoral arteries were cannulated with PE10 tubing (Becton Dickinson, Sparks, MD). The right arterial catheter was connected to a pressure monitor (Grass, West Warwick, RI) to follow mean arterial pressure (MAP). Blood was withdrawn over 15 min via the left femoral artery catheter to reduce the MAP to 35 mmHg. Blood was withdrawn and returned to the animal as needed to maintain a MAP of 35 ± 5 mmHg. Sham animals were cannulated but were not subjected to hemorrhage. At the end of the shock period (90 min), the mice were resuscitated with the shed blood plus two times that volume of Ringer's lactate solution infused slowly over 30 min.
Experimental design
HB-EGF KO and WT mice were randomly assigned to the following groups (n=4 animals per group); 1) experimental group: animals were subjected to HS/R, and sacrificed at 1, 3 and 6 h after the initiation of resuscitation; 2) sham operated controls: animals were anesthetized and catheterized; but no hemorrhage was induced. Small intestines from animals of each group were obtained for histologic examination at 1, 3, and 6 h timepoints.
Histology
For histologic examination, samples of ileum were fixed in 10% formaldehyde for 24 h and then embedded in paraffin. 5-μm thick sections were stained with hematoxylin and eosin. Histologic injury was graded blindly using a standardized histologic injury scoring system according to Murao et al.15 with modifications as follows: Grade 0: no injury; Grade 1: vacuolization at the tips of villi; Grade 2: vacuolization at the upper one third of villi; Grade 3: vacuolization at the upper two thirds of villi; Grade 4: presence of a subepithelial space; Grade 5: mucosal ulceration (Figure 1).
Figure 1.
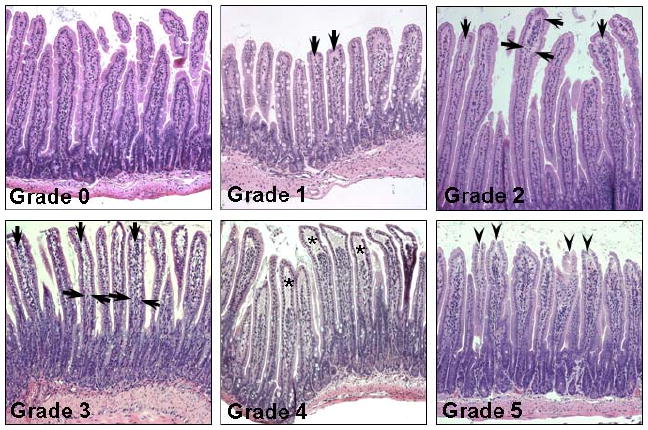
Grading system for histological injury after HS/R. Grade 0, no injury; Grade 1, vacuolization at the tips of villi; Grade 2, vacuolization at the upper one third of villi; Grade 3, vacuolization at the upper two thirds of villi; Grade 4, presence of a subepithelial space; Grade 5, mucosal ulceration. Arrows indicate areas of vacuolization; asterisks indicate subepithelial spaces, arrowheads indicate the disrupted tips of the villi.
TUNEL staining
Terminal deoxynucleotidyl transferase-mediated deoxyuridine 5-triphosphate nick-end labeling (TUNEL) staining was used to detect fragmented DNA in situ in paraffin sections using a commercially available test kit (ApopTag Red In Situ Apoptosis Detection; Chemicon International, Inc., CA) following the manufacturer's instructions. Six sections of terminal ileum for each animal were examined blindly under 40 × magnification. In each section, positively stained enterocytes and total enterocytes in the villi and crypts were counted. Typically, 4000-6000 total enterocytes were counted for each animal. The apoptosis index was defined as the percent of positively stained enterocytes / 100 enterocytes.
Determination of intestinal permeability
Small intestinal mucosal barrier function was assessed by using the ex vivo isolated everted sac method as described, with some modifications.16 Briefly, 6 cm long distal jejunal everted gut sacs were prepared in ice-cold modified Krebs-Henseleit bicarbonate buffer (KHBB, pH 7.4, 10 mM Hepes / 137 mM NaCl / 5.5 mM KCl / 4.2 mM NaHCO3 / 0.3 mM Na2HPO4 / 0.4 mMKH2PO4 / 0.4 mM MgSO4 / 0.5 mM MgCl2 / 1.3 mM CaCl2 / 19.5 mM glucose). Fluorescein-isothiocyanate dextran (Mr 4000 Da; FD4) was used as a permeability probe. The everted gut sacs were gently distended by injecting 0.4 ml of KHBB and suspending the sacs in a 50 ml-beaker containing 40 ml of KHBB with added FD4 (100 μg/ml) for 30 min. The incubation medium in the beaker was maintained at a temperature of 37°C and was continuously bubbled with a gas mixture containing 95% O2 and 5% CO2. A 1 ml sample was taken from the beaker at the beginning of the incubation to determine the initial FD4 concentration of the mucosal side. After the 30 min incubation, the fluid was aspirated from the inside of the sac to determine the FD4 concentration of the serosal side. The length and diameter of each gut sac was measured. Serosal and mucosal samples were centrifuged for 10 min at 1000 g at 4°C. Fluorescence of 100 μl of supernatant was measured using an LS-50 fluorescence spectrophotometer (Perkin-Elmer, Palo Alto, CA) at an excitation wavelength of 492 nm (slit width, 2.5 nm) and an emission wavelength of 515 nm (slit width, 10 nm). Gut permeability was expressed as the mucosal-to-serosal clearance of FD4 as follows16: FD4 clearance (nL/min/cm2) = FD4ser × (Original 0.4 ml +Absorbed Volume) × 30-1× FD4muc-1 × (πDL)-1
Statistical analyses
To take into consideration repeated measurements from the same animals, the differences in injury scores, apoptosis indices, and intestinal permeability between different groups under sham control and hemorrhage shock conditions at each time point were tested using linear mixed effects models. The Holms procedure was used to correct for multiple endpoints and p<0.05 was considered significant after adjustment for multiple comparisons.
Results
HB-EGF gene deletion is associated with increased structural intestinal injury in mice subjected to HS/R
To evaluate the role of endogenous HB-EGF in tolerance to intestinal injury, HB-EGF KO and WT mice were subjected to HS/R. All mice (KO and WT) subjected to HS/R had significantly increased histologic injury scores at 1, 3 and 6 h of reperfusion compared to mice subjected to sham surgery only (Figure 2). HB-EGF KO mice had significantly increased histologic injury compared to WT mice at 3 and 6 h of resuscitation (mean injury grade 4.5 ± 1.0 vs. 2.75 ± 0.5 at 3 h of resuscitation, p=0.0009). Thus, HB-EGF KO mice have more serious histologic intestinal injury compared to WT mice upon recovery from hemorrhagic shock, indicating that endogenous HB-EGF gene expression is essential for preservation of structural integrity after HS/R.
Figure 2.
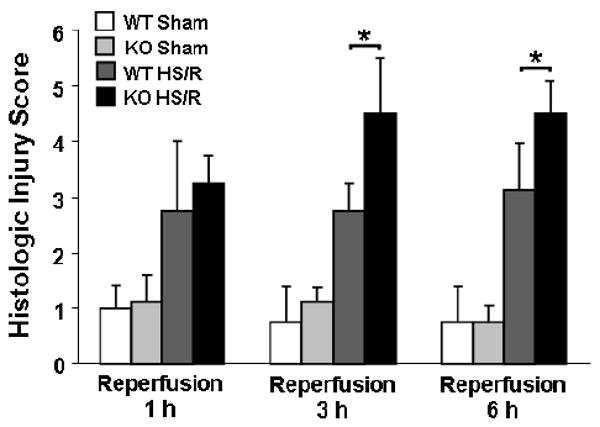
HB-EGF gene deletion is associated with higher intestinal injury scores in mice subjected to HS/R. HB-EGF KO and WT mice were subjected to hemorrhagic shock (MAP of 35 ± 5 mmHg) for 90 minutes followed by reperfusion for 1, 3 and 6 hours. Histologic injury in the ileum was graded using the scoring system described in Figure 1. *p<0.05.
HB-EGF gene deletion is associated with increased enterocyte apoptosis in mice subjected to HS/R
Since apoptosis is a major mode of IEC death induced by I/R injury, we next evaluated the effect of endogenous HB-EGF gene deletion on enterocyte apoptosis using TUNEL staining of histologic sections to calculate the apoptosis index (defined as the percent of positively stained enterocytes / 100 enterocytes). All mice (KO and WT) subjected to HS/R had significantly increased apoptosis indices at 1, 3 and 6 h of resuscitation compared to mice subjected to sham surgery only (Figure 3). HB-EGF KO mice had significantly increased apoptosis indices compared to WT mice at 1, 3 and 6 h of resuscitation (mean apoptosis index 6.84 ± 1.95 vs. 3.24 ± 1.00 at 3 h of resuscitation, p<0.0001). The increased IEC apoptosis in HB-EGF KO mice after HS/R supports a role for endogenous HB-EGF gene expression in protection of enterocytes from apoptosis after injury.
Figure 3.
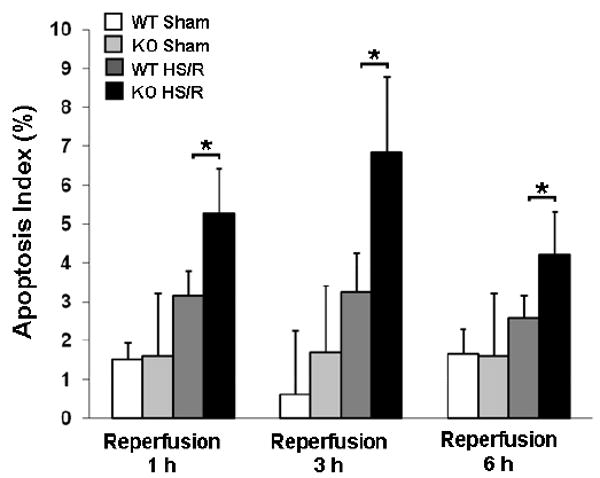
HB-EGF gene deletion is associated with increased apoptosis in mice subjected to HS/R. HB-EGF KO and WT mice were subjected to hemorrhagic shock (MAP of 35 ± 5 mmHg) for 90 minutes followed by reperfusion for 1, 3 and 6 hours. Histologic sections of terminal ileum were subjected to TUNEL staining, positively stained enterocytes and total enterocytes in the villi and crypts were counted, and apoptosis indices calculated as the percent of positively stained enterocytes / 100 enterocytes. *p<0.05.
HB-EGF gene deletion is associated with intestinal barrier dysfunction in mice subjected to HS/R
Since increased enterocyte apoptosis may lead to gut barrier dysfunction after intestinal injury, we next evaluated the effect of endogenous HB-EGF gene deletion on the functional gut permeability barrier, as determined by mucosal-to-serosal unidirectional clearance to FD4 using the everted gut sac method. All mice (KO and WT) subjected to HS/R had significantly increased intestinal permeability at 1, 3 and 6 h of resuscitation compared to mice subjected to sham surgery only (Figure 4). HB-EGF KO mice had significantly increased mucosal permeability compared to WT mice at 1, 3 and 6 h of resuscitation (FD4 clearance 78 ± 18.91 vs. 47.75 ± 8.06 nl/min/cm2 at 3 h of resuscitation, p<0.05). This indicates that HB-EGF KO mice have increased intestinal permeability after HS/R, and that endogenous HB-EGF gene expression is essential for the preservation of a functional gut barrier during recovery of the intestine from injury.
Figure 4.
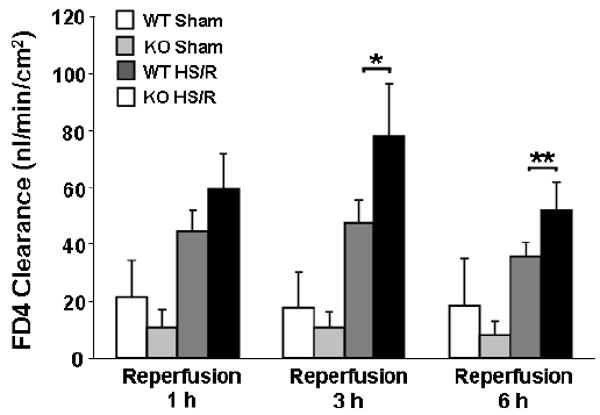
HB-EGF gene deletion is associated with increased intestinal permeability in mice subjected to HS/R. HB-EGF KO and WT mice were subjected to hemorrhagic shock (MAP of 35 ± 5 mmHg) for 90 min followed by reperfusion for 1, 3 and 6 h. Intestinal permeability was determined by FD4 clearance using the everted gut sac method. *p<0.05, **p=0.05.
Discussion
Our results demonstrate that endogenous HB-EGF is important in intestinal barrier function after HS/R. Since HB-EGF KO mice have deletion of HB-EGF only, with preservation of other EGF family members, our results also show that the effects of loss of HB-EGF on gut barrier function cannot be compensated for by the presence of other EGF family members.
HB-EGF has been shown to play important physiological roles in the process of healing after injury. The function of endogenous HB-EGF in skin wound healing has been investigated using a keratinocyte-specific HB-EGF KO mouse model, in which wound closure was markedly impaired in the KO mice.17 This was confirmed in vitro, where functional blocking of endogenous HB-EGF using HB-EGF neutralizing antibodies caused a significant reduction in keratinocyte migration resulting in delayed wound healing.18 Others have found that liver regeneration was delayed in HB-EGF KO mice after two-thirds partial hepatectomy.19 We have shown that HB-EGF KO mice have impaired intestinal restitution and angiogenesis, and decreased survival, after intestinal I/R due to superior mesenteric artery occlusion.10 Collectively, these studies suggest that endogenous HB-EGF plays a key role in healing, a role that cannot be compensated for by the presence of other EGF family members.
Compared with other injury models, the HS/R mouse model has some unique characteristics. Among the internal organs, the intestine is probably the most sensitive to I/R injury.1 Interruption of the blood supply to the intestine results in ischemic injury which rapidly damages this metabolically active tissue. In addition, restoration of blood flow to the ischemic intestine initiates a cascade of events known as reperfusion injury, which may lead to even more serious injury than the original ischemic insult.1, 2 When the intestine is subjected to HS/R, the earliest lesion of the intestinal mucosa seen by light microscopy following hypoxic shock is enterocyte cytoplasmic vacuolization, indicating that the cellular organelles are affected by hypofusion.15, 20 Enterocyte vacuolization is followed by structural damage, with injury to the basal portion of the villi and in the intestinal crypts appearing much later than injury to the distal villi.21 In the current study, we found that HB-EGF KO mice sustained more serious intestinal injury than HB-EGF WT mice after HS/R, indicating that endogenous HB-EGF gene expression is involved in the structural recovery of the intestinal barrier after injury.
Apoptosis is a major mode of IEC death during I/R injury. In the early stage of I/R, over 80% of the detached cells exhibited characteristic morphological features of apoptosis, with only 20% demonstrating necrosis.22 In previous studies using mouse muscle myoblast C2C12 cells, blocking of endogenous HB-EGF with neutralizing antibodies significantly enhanced sensitivity to apoptosis induced by hypoxic stress.23 We have previously shown that addition of HB-EGF to pro-inflammatory cytokine stimulated IEC in vitro increases resistance to apoptosis.24 Our current results reveal that HB-EGF KO mice have significantly increased apoptosis indices compared to WT mice after HS/R, suggesting that endogenous HB-EGF plays an anti-apoptotic role after intestinal injury due to HS/R.
We have demonstrated that mucosal hyperpermeability is detectable 1 h after resuscitation in HB-EGF KO mice exposed to HS/R, and showed that this abnormality persists until at least 6 h after resuscitation. This significantly increased gut permeability indicates that endogenous HB-EGF plays an important role in the preservation of intestinal barrier function after injury.
In conclusion, our results show that HB-EGF is essential for preservation of gut barrier function after HS/R, and that lack of HB-EGF expression cannot be compensated for by the presence of other EGF family members. These findings support the clinical use of HB-EGF to protect the intestines from disease states associated with intestinal hypoperfusion injury.
Footnotes
Financial Disclosures: This work was supported by a grant from the National Institutes of Health R01 GM61193 (GEB). Dr. Besner has a licensing agreement, scientific research agreement and consulting agreement with Trillium Therapeutics, Inc. (Toronto, Canada). Please note that the work presented here is not related in any way to the aforementioned agreements. Drs. Zhang and Radulescu have no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matheson JP, Wilson AM, Garrison RN. Regulation of intestinal blood flow. J Surg Res. 2000;93:182–96. doi: 10.1006/jsre.2000.5862. [DOI] [PubMed] [Google Scholar]
- 2.Seal J, Gewertz B. Vascular dysfunction in ischemia-reperfusion injury. Ann Vasc Surg. 2005;19:572–84. doi: 10.1007/s10016-005-4616-7. [DOI] [PubMed] [Google Scholar]
- 3.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage derived heparin-binding growth factor. Cell Regul. 1990;1:811–9. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higashiyama S, Abraham JA, Miller J, Fiddes JC, Klagsbrun M. A heparin-binding growth factor secreted by macrophage-like cells that is related to EGF. Science. 1991;251:936–9. doi: 10.1126/science.1840698. [DOI] [PubMed] [Google Scholar]
- 5.Nishi E, Klagsbrun M. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) is a mediator of multiple physiological and pathological pathways. Growth Factors. 2004;22:253–60. doi: 10.1080/08977190400008448. [DOI] [PubMed] [Google Scholar]
- 6.Xia G, Rachfal AW, Martin AE, Besner GE. Upregulation of endogenous heparin-binding EGF-like growth factor (HB-EGF) expression after intestinal ischemia/reperfusion injury. J Invest Surg. 2003;16:57–63. [PubMed] [Google Scholar]
- 7.Kuhn MA, Xia G, Mehta VB, Glenn S, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) decreases oxygen free radical production in vitro and in vivo. Antioxid Redox Signal. 2002;4:639–46. doi: 10.1089/15230860260220148. [DOI] [PubMed] [Google Scholar]
- 8.Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434–9. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- 9.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–25. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 10.El-Assal ON, Paddock H, Marquez A, Besner GE. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg. 2008;43:1182–90. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehta VB, Zhou Y, Radulescu A, Besner GE. HB-EGF stimulates eNOS expression and nitric oxide production and promotes eNOS dependent angiogenesis. Growth Factors. 2008;26:301–15. doi: 10.1080/08977190802393596. [DOI] [PubMed] [Google Scholar]
- 12.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142(2):234–42. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41(1):144–9. doi: 10.1016/j.jpedsurg.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 14.Jackson LF, Qiu TH, Sunnarborg SW, Chang A, Zhang C, Patterson C, et al. Defective valvulogenesis in HB-EGF and TACE-null mice is associated with aberrant BMP signaling. The EMBO J. 2003;22:2704–16. doi: 10.1093/emboj/cdg264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murao Y, Hata M, Ohnishi K, Okuchi K, Nakajima Y, Hiasa Y, et al. Hypertonic saline resuscitation reduces apoptosis and tissue damage of the small intestine in a mouse model of hemorrhagic shock. Shock. 2003;20:23–8. doi: 10.1097/01.shk.0000078832.57685.6c. [DOI] [PubMed] [Google Scholar]
- 16.Liaudet L, Soriano FG, Szabó E, Virág L, Mabley JG, Salzman AL, et al. Protection against hemorrhagic shock in mice genetically deficient in poly(ADP-ribose)polymerase. Proc Natl Acad Sci USA. 2000;97:10203–8. doi: 10.1073/pnas.170226797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shirakata Y, Kimura R, Nanba D, Iwamoto R, Tokumaru S, Morimoto C, et al. Heparin-binding EGF-like growth factor accelerates keratinocyte migration and skin wound healing. J Cell Sci. 2005;118:2363–70. doi: 10.1242/jcs.02346. [DOI] [PubMed] [Google Scholar]
- 18.Koivisto L, Jiang G, Häkkinen L, Chan B, Larjava H. HaCaT keratinocyte migration is dependent on epidermal growth factor receptor signaling and glycogen synthase kinase-3alpha. Exp Cell Res. 2006;312:2791–805. doi: 10.1016/j.yexcr.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Mitchell C, Nivison M, Jackson LF, Fox R, Lee DC, Campbell JS, et al. Heparin-binding epidermal growth factor-like growth factor links hepatocyte priming with cell cycle progression during liver regeneration. J Biol Chem. 2005;280:2562–8. doi: 10.1074/jbc.M412372200. [DOI] [PubMed] [Google Scholar]
- 20.Henics T, Wheatley DN. Cytoplasmic vacuolation, adaptation and cell death: A view on new perspectives and features. Biol Cell. 1999;91:485–98. doi: 10.1016/s0248-4900(00)88205-2. [DOI] [PubMed] [Google Scholar]
- 21.Morini S, Yacoub W, Rastellini C, Gaudio E, Watkins SC, Cicalese L. Intestinal microvascular patterns during hemorrhagic shock. Dig Dis Sci. 2000;45:710–22. doi: 10.1023/a:1005491509832. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda H, Suzuki Y, Suzuki M, Koike M, Tamura J, Tong J, et al. Apoptosis is a major mode of cell death caused by ischemia and ischemia/reperfusion injury to the rat intestinal epithelium. Gut. 1998;42:530–7. doi: 10.1136/gut.42.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horikawa M, Higashiyama S, Nomura S, Kitamura Y, Ishikawa M, Taniguchi N. Upregulation of endogenous heparin-binding EGF-like growth factor and its role as a survival factor in skeletal myotubes. FEBS Lett. 1999;459:100–4. doi: 10.1016/s0014-5793(99)01213-2. [DOI] [PubMed] [Google Scholar]
- 24.Michalsky MP, Kuhn A, Mehta V, Besner GE. Heparin-binding EGF-like growth factor decreases apoptosis in intestinal epithelial cells in vitro. J Pediatr Surg. 2001;36:1130–5. doi: 10.1053/jpsu.2001.25730. [DOI] [PubMed] [Google Scholar]


