Table 2.
Rh(I)-Catalyzed Isomerizations of Bicyclo[1.1.0]butanes 4
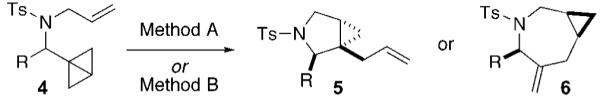 | |||
|---|---|---|---|
| entry | substrate | method Aa | method Bb |
| 1 | 4a, R = 3,5-(MeO)2C6H3 | 5a, 67% | 6a, 87% |
| 2 | 4b, R = 4-ClC6H4 | 5b, 55% | 6b, 80% |
| 3 | 4c, R = 2-furyl | 5c, 75% | 6c, 58% |
| 4 | 4d, R = BnOCH2 | 5d, 63% | 6d, 68% |
| 5 | 4e, R = cyclohexyl | 5e, 65% | 6e, 75% |
Method A: [Rh(=)2Cl]2 (5 mol %), Ph3P (10 mol %), PhMe (0.05 M), 110 °C.
Method B: [Rh(CO)2Cl]2 (5 mol %), dppe (10 mol %), PhMe (0.05 M), 110 °C.
