Abstract
The precise cell-cycle alternation of S phase and mitosis is controlled by alternating competence of nuclei to respond to S-phase-inducing factors [1]. Nuclei acquire competence to replicate at the low point in cyclin-dependent kinase (Cdk) activities that follows mitotic destruction of cyclins. The elevation of Cdk activity late in G1 is thought to drive cells into S phase and to block replicated DNA from re-acquiring replication competence [2]. Whereas mitosis is normally required to eliminate the cyclins prior to another cycle of replication, experimental elimination of Cdk activity in G2 can restore competence to replicate [3–6]. Here, we examine the roles of Cdks in the endocycles of Drosophila [7]. In these cycles, rounds of discrete S phases without intervening mitoses result in polyteny. Cyclins A and B are lost in cells as they enter endocycles [8,9], and pulses of Cyclin E expression drive endocycle S phases [10–12]. To address whether oscillations of Cyclin E expression are required for endocycles, we expressed Cyclin E continuously in Drosophila salivary glands. Growth of the cells was severely inhibited, and a period of DNA replication was induced but further replication was inhibited. This replication inhibition could be overcome by the kinase inhibitor 6-dimethylaminopurine (6-DMAP), but not by expression of subunits of the transcription factor E2F. These results indicate that endocycle S phases require oscillations in Cdk activity, but, in contrast to oscillations in mitotic cells, these occur independently of mitosis.
Results and discussion
To test whether fluctuations in Cyclin E levels are required for endoreplication, we induced continuous Cyclin E expression in Drosophila salivary glands. A transgene expressing the transcriptional activator Gal4 in the salivary gland (43B; provided by B. Noll and N. Perrimon) was used to drive expression of a second transgene encoding Cyclin E under the control of the Gal4 upstream activating sequence (UAS–Cyclin E; provided by H. Richardson). Ectopic Cyclin E mRNA first accumulated in embryos in late stage 14 (Figure 1a,b); at this stage, the salivary gland cells had undergone two consecutive rounds of replication following their last mitosis [7,13] and were in a gap phase of the endocycle. This induced Cyclin E expression continued throughout larval development (Figure 1c,d). The continuous expression of Cyclin E reduced greatly the size of the third instar salivary glands (Figure 1e,f) without noticeably compromising larval growth or survival (data not shown). The salivary gland nuclei were much smaller and had about 40-fold less DNA than wild-type salivary glands, as assessed by micro-fluorometric measurement of Hoechst 33258 staining (data not shown). These results suggest that continuous expression of Cyclin E severely inhibits both the degree of endoreplication and the overall growth of larval salivary glands.
Figure 1.
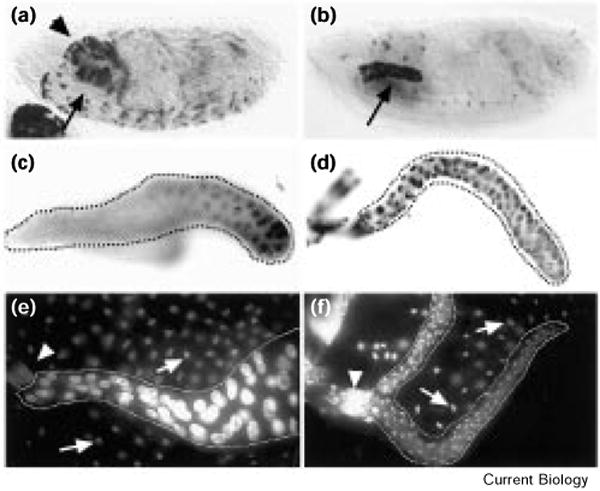
Continuous Cyclin E expression begins in embryonic salivary glands and continues throughout larval development. (a,b) Embryos and (c,d) salivary glands (outlined) of early third instar larvae were hybridized with a digoxigenin-labeled Cyclin E RNA probe that was visualized using alkaline-phosphatase-conjugated anti-digoxigenin antibodies. Cyclin E RNA accumulated in the salivary glands (arrows) of 43B-UAS–Cyclin E embryos (b) by stage 15, at which time wild-type (Sevelin) embryos (a) do not express Cyclin E in the salivary glands. The arrowhead in (a) indicates labeling in the brain, which is somewhat out of focus in (b). (c) A fraction of the cells of the larval salivary gland express Cyclin E RNA in the wild-type (yw67) salivary glands, whereas all of the cells of the 43B-UAS–Cyclin E glands (d) express Cyclin E. (e) Late third instar wild-type and (f) 43B-UAS–Cyclin E salivary glands (outlined by dotted lines) stained with 1 μg/ml Hoechst 33258. Whereas the salivary glands normally increase in size dramatically during the third instar, the salivary glands of the 43B-UAS–Cyclin E larvae remain small. Arrowheads indicate the ring of diploid cells forming the imaginal ring. Arrows indicate the nuclei of the fat body; cells of the fat body (which are not expressing Cyclin E continuously in these experiments) also endoreplicate but to a lesser extent than cells of the wild-type salivary gland. Note the relative size of the fat body nuclei compared to the wild-type and 43B-UAS–Cyclin E salivary gland nuclei.
Incorporation of bromodeoxyuridine (BrdU) revealed that DNA synthesis was induced at the onset of ectopic Cyclin E expression (data not shown), consistent with the ability of Cyclin E to drive endocycling cells into S phase ([10] and T.T. Su and P.H.O'F., unpublished observations). In contrast, subsequent BrdU incorporation into the salivary glands of 43B-UAS–Cyclin E larvae was largely inhibited in all larval instars (Figure 2a,b, and data not shown). These results support the hypothesis that Cyclin E levels must fluctuate for S phase to occur in successive endocycles.
Figure 2.
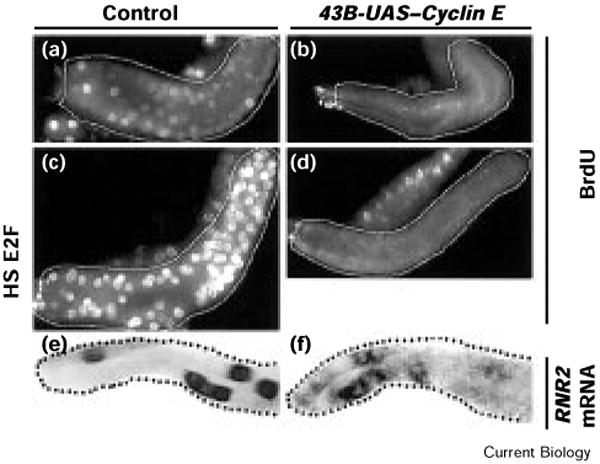
Continuous Cyclin E expression in Drosophila salivary glands causes a prolonged period of replication inhibition in larvae. (a,b) Early third instar larval salivary glands (outlined) were dissected and cultured with 1 mg/ml BrdU for 30 min. The incorporated nucleotide was detected by immunofluorescent staining. (a) Wild-type salivary glands showed BrdU incorporation in a subset of nuclei. Whereas little or no incorporation is seen in 43B-UAS–Cyclin E salivary glands (b), we did detect occasional small dots of BrdU incorporation and rare nuclei with more complete labeling (not evident in this figure). We suggest that these dots of BrdU incorporation result from the firing of isolated replication origins: if the replication block created by the UAS–Cyclin E and 43B transgenes is imperfect, then a failure of the block may result in the sporadic assembly of replication components at an origin followed by local replication. (c,d) Salivary glands (outlined) from early third instar larvae that carry heat-shock-inducible (HS) transgenes for E2F and DP were labeled by culturing dissected glands with BrdU for 30 min. (c) Induction of the subunits of E2F (E2F and DP) in a wild-type background causes an increase in BrdU incorporation but not in (d) the salivary glands of 43B-UAS–Cyclin E larvae that express Cyclin E continuously. Larvae were heat shocked at 37°C for 1 h, allowed to recover for 2 h, and dissected and labeled in Schneider's tissue culture medium with 1 mg/ml BrdU. (e,f) Early third instar salivary glands were hybridized with a digoxigenin-labeled probe for RNR2 RNA and the probe visualized immunohistochemically. RNR2 is a target of E2F, and provides an indication of E2F activity. In both (e) wild-type and (f) 43B-UAS–Cyclin E salivary glands, RNR2 is expressed in a subset of the cells of the gland.
Although we have been unable to monitor Cyclin E protein levels directly by antibody staining, the replication inhibition caused by continuous expression of Cyclin E presumably reflects continuous Cyclin-E-associated kinase activity. If Cdk activity must indeed fluctuate for S phase to occur in successive endocycles, then a transient inhibition of kinase activity in these cells might be sufficient to allow replication once Cyclin E kinase levels are restored. To test this, we pulse-treated 43B-UAS–Cyclin E salivary glands with 6-DMAP [14], an inhibitor of kinase activity, and tested for BrdU incorporation. We observed a dramatic increase in the amount of BrdU incorporated following treatment with 6-DMAP (Figure 3). This result is consistent with the premise that Cdk activity must fluctuate in these cells to allow multiple rounds of S phase, and demonstrates that DNA replication in salivary gland cells is not irreversibly compromised by the continuous expression of Cyclin E.
Figure 3.
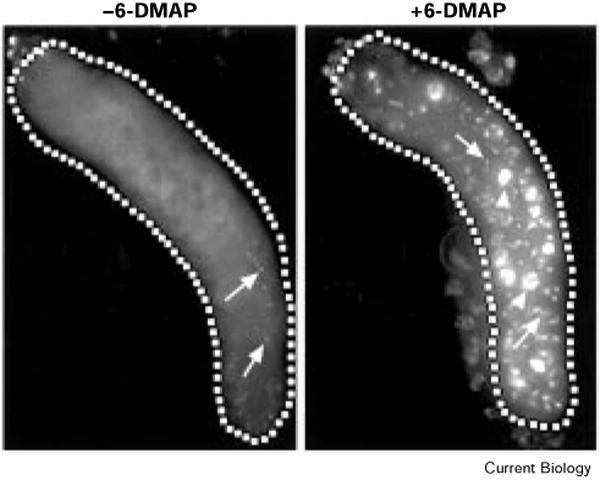
The kinase inhibitor 6-DMAP causes an additional period of BrdU incorporation in 43B-UAS–Cyclin E larval salivary glands. Salivary glands were dissected from second instar 43B-UAS–Cyclin E larvae and incubated for 30 min in tissue culture medium with or without 10 mM 6-DMAP. The glands were then washed six times for 5 min with medium free of 6-DMAP and labeled for 30 min in culture medium with BrdU. (a) Untreated glands show only some small dots of BrdU incorporation (arrows). (b) Treatment with 6-DMAP resulted in BrdU incorporation in both dots (arrows) and entire nuclei (arrowheads). BrdU was detected using rhodamine-conjugated secondary antibodies and the images captured using a CCD (charge-coupled device) camera.
Whereas the block to re-replication in mitotic cells is generally thought to affect replication components directly, there are other possibilities illustrated by the actions of cyclin A. In Drosophila, Cyclin A plays the key role in blocking replication: its elimination is sufficient to convert mitotic cycles to endocycles [6], and its ectopic expression in endocycling cells blocks endocycle S phases (F. Sprenger, N. Yakubovich, and P.H.O'F., unpublished observations). In mammals, cyclin A–Cdk2 can inhibit the S-phase transcription factor E2F [15]. As Drosophila E2F plays a major role in the embryonic endocycle S phase [16], Cyclin A might block replication indirectly, by preventing the activation of E2F. To test whether E2F is involved in the Cyclin-E-induced block to re-replication, we examined the effect of continuous Cyclin E expression on the accumulation of E2F-dependent transcripts, and tested whether expression of the subunits of E2F could bypass the re-replication block. The expression of RNR2, a target gene of E2F [16,17], was altered by continuous Cyclin E expression — there appeared to be more cells, on average, expressing RNR2 within each 43B-UAS–Cyclin E salivary gland than in wild-type glands, and the level of RNR2 expression within each Cyclin-E-expressing cell appeared lower than in wild-type cells. There was, nevertheless, significant RNR2 accumulation in 43B-UAS–Cyclin E salivary glands (Figure 2e,f). It thus appears unlikely that the S-phase block caused by continuous Cyclin E expression occurs via E2F inactivation.
To further address the role of E2F in the Cyclin-E-induced block to replication, we monitored the ability of E2F to induce S phase in 43B-UAS–Cyclin E salivary glands. Heat-shock induction of the subunits of E2F (E2F and DP) induced near-uniform incorporation of BrdU in wild-type endoreplicating cells (Figure 2c). Induction of the subunits of E2F in 43B-UAS–Cyclin E salivary glands, however, had no such effect (Figure 2d). Together, these results suggest that the S phase block created by continuous Cyclin E expression acts either downstream of E2F activation or on a parallel pathway that is required for S phase. As the Cyclin A block to re-replication appears to be bypassed by the expression of E2F subunits, this result suggests that there are two mechanisms of S-phase inhibition, one upstream and one downstream of, or parallel to, E2F activation.
The results presented here demonstrate that continuous ectopic Cyclin E expression inhibits S phase in endocycling cells. We infer that Cyclin E expressed from the endogenous gene has the same activity, but we cannot entirely exclude the possibility that the transgene expresses a greater activity. In any case, it is clear that Cyclin E, like Cyclin A, can have both a positive and a negative role in the control of DNA replication. The demonstration of these two activities supports a general model in which fluctuations in Cdk activity are required for DNA replication in successive cell cycles [2,5,6], and suggests that there is an oscillation in replication competence in the endocycle as there is in the mitotic cycle. The change in the program of expression of the different cyclins in endocycles enables the fluctuations in Cdk activity to be independent of mitosis, however (Figure 4).
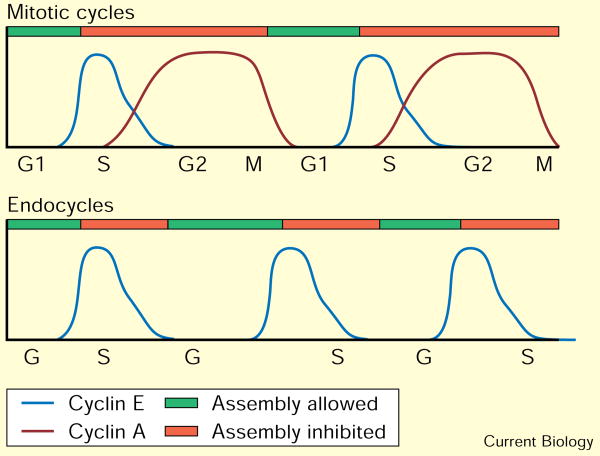
Figure 4.
A model to explain the control of S phase in mitotic cycles and endocycles. We summarize here previous proposals [2,5,6,10,11,15] for the coordination of DNA replication with mitotic cycles and endocycles, with emphasis on the newly demonstrated ability of cyclin E to inhibit re-replication. In mitotic cells, the absence of Cdk activity allows the assembly of replication origins during the G1 phase. Because Cdks are required in order for assembled origins to fire, DNA replication does not begin until Cdk activity appears in late G1 phase, provoking entry into S phase. Once in S phase, re-assembly of fired origins is inhibited by Cdk activity, which is present until the G2 cyclins (specifically cyclin A) are degraded during mitosis. In endocycles, fluctuations in Cdk activity cause a similar cycle of origin assembly, origin firing, and prevention of reassembly, but here the ability to reassemble origins does not rely on passage through mitosis. Instead, the G2 cyclins are not expressed in these cells, and the prevention of reassembly is carried out by cyclin-E-associated kinase activity, which fluctuates independently of mitosis.
The ability of Cyclin E to block re-replication presents a paradox. Cyclin-E-associated kinase activity is high throughout the early cell cycles of Drosophila embryogenesis [6]. Apparently, either Cyclin E lacks S-phase-inhibitory activity in these early cell cycles, or mitosis can override the Cyclin-E-mediated inhibition and restore replication competence even in the continuing presence of Cyclin E. Experiments in Xenopus cell extracts have led to the suggestion that the mitotic event that allows re-replication is the breakdown of the nuclear envelope [18].
One potential mechanism has been suggested by Hua et al. [19], who point out that when the nuclear envelope breaks down during mitosis, the local concentration of cyclin E (a nuclear protein) near the chromosomes decreases. Thus, although the overall levels of cyclin E activity within the embryo remain constant during mitosis, the levels of cyclin E at replication origins may fall below the level required to prevent re-replication. We find, however, that increased expression of Cyclin E in Drosophila does not block BrdU incorporation in early mitotic cycles (P.J.F. and P.H.O'F., unpublished observations). This argues against the idea that the mechanism of restoration of replication competence relies on modest changes in the concentration of Cyclin E. Future experiments addressing the regulation of Cyclin E during these early mitotic cycles, as well as during endocycles, should shed additional light on the regulation of both Cdk activity and S phase during the cell cycle.
Acknowledgments
We thank B. Noll, N. Perrimon, and H. Richardson for fly stocks, C. Bunker for digoxigenin-labeled RNA probes, and members of the O'Farrell lab, especially N. Serrano and P. DiGregorio, for comments and discussion. This work was supported by a NIH grant to P.H.O'F. P.J.F. was supported by a NSF Graduate Research Fellowship.
References
- 1.Rao PN, Johnson RT. Mammalian cell fusion: studies on the regulation of DNA synthesis and mitosis. Nature. 1970;225:159–164. doi: 10.1038/225159a0. [DOI] [PubMed] [Google Scholar]
- 2.Su TT, Follette PJ, O'Farrell PH. Qualifying for the license to replicate. Cell. 1995;81:825–828. doi: 10.1016/0092-8674(95)90000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broek D, Bartlett R, Crawford K, Nurse P. Involvement of p34cdc2 in establishing the dependency of S phase on mitosis. Nature. 1991;349:388–393. doi: 10.1038/349388a0. [DOI] [PubMed] [Google Scholar]
- 4.Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic B cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- 5.Dahmann C, Diffley JF, Nasmyth KA. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- 6.Sauer K, Knoblich JA, Richardson H, Lehner CF. Distinct modes of cyclin E/cdc2c kinase regulation and S-phase control in mitotic and endoreduplication cycles of Drosophila embryogenesis. Genes Dev. 1995;9:1327–1339. doi: 10.1101/gad.9.11.1327. [DOI] [PubMed] [Google Scholar]
- 7.Smith AV, Orr-Weaver TL. The regulation of the cell cycle during Drosophila embryogenesis: the transition to polyteny. Development. 1991;112:997–1008. doi: 10.1242/dev.112.4.997. [DOI] [PubMed] [Google Scholar]
- 8.Lehner CF, O'Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lehner CF, O'Farrell PH. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knoblich JA, Sauer K, Jones L, Richardson H, Saint R, Lehner CF. Cyclin E controls S phase progression and its down-regulation during Drosophila embryogenesis is required for the arrest of cell proliferation. Cell. 1994;77:107–120. doi: 10.1016/0092-8674(94)90239-9. [DOI] [PubMed] [Google Scholar]
- 11.Lilly MA, Spradling AC. The Drosophila endocycle is controlled by cyclin E and lacks a checkpoint ensuring S-phase completion. Genes Dev. 1996;10:2514–2526. doi: 10.1101/gad.10.19.2514. [DOI] [PubMed] [Google Scholar]
- 12.Richardson HE, O'Keefe LV, Reed SI, Saint R. A Drosophila G1-specific cyclin E homolog exhibits different modes of expression during embryogenesis. Development. 1993;119:673–690. doi: 10.1242/dev.119.3.673. [DOI] [PubMed] [Google Scholar]
- 13.Sigrist S, Lehner CF. Drosophila fizzy-related down-regulates mitotic cyclins and is required for cell proliferation arrest and entry into endocycles. Cell. 1997;90:671–681. doi: 10.1016/s0092-8674(00)80528-0. [DOI] [PubMed] [Google Scholar]
- 14.Vesely J, Havlicek L, Strnad M, Blow JJ, Donella-Deana A, Pinna L, et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur J Biochem. 1994;224:771–786. doi: 10.1111/j.1432-1033.1994.00771.x. [DOI] [PubMed] [Google Scholar]
- 15.Dynlacht BD, Flores O, Lees JA, Harlow E. Differential regulation of E2F transactivation by cyclin/cdk2 complexes. Genes Dev. 1994;8:1772–1786. doi: 10.1101/gad.8.15.1772. [DOI] [PubMed] [Google Scholar]
- 16.Duronio RJ, O'Farrell PH. Developmental control of the G1 to S transition in Drosophila: cyclin E is a limiting downstream target of E2F. Genes Dev. 1995;9:1456–1468. doi: 10.1101/gad.9.12.1456. [DOI] [PubMed] [Google Scholar]
- 17.Duronio RJ, O'Farrell PH. Developmental control of a G1-S transcriptional program in Drosophila. Development. 1994;120:1503–1515. doi: 10.1242/dev.120.6.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blow JJ, Laskey RA. A role for the nuclear envelope in controlling DNA replication within the cell cycle. Nature. 1988;332:546–548. doi: 10.1038/332546a0. [DOI] [PubMed] [Google Scholar]
- 19.Hua XJ, Yan H, Newport J. A role for Cdk2 kinase in negatively regulating DNA replication during S phase of the cell cycle. J Cell Biol. 1997;137:183–192. doi: 10.1083/jcb.137.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]