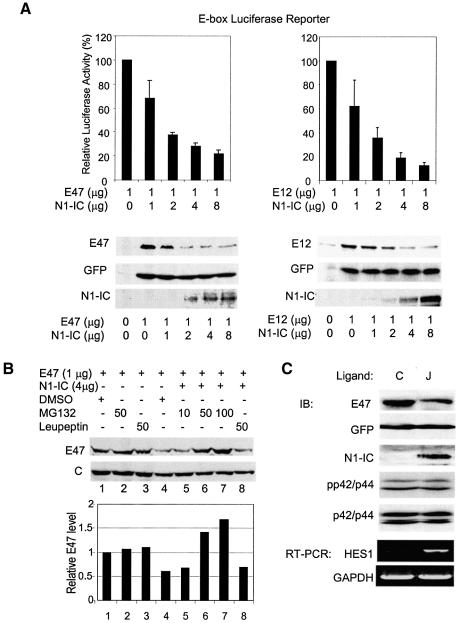
Fig. 1. N1-IC inhibits E47 and E12 transcriptional activity by accelerating their degradation. (A) Dose responses. NIH3T3 cells were transiently transfected with 5 µg, 1 µg each of E-box luciferase and β-galactosidase reporter plasmids along with 1 µg of E47 or E12 plus increasing amounts of N1-IC expressing constructs. Luciferase activities were assayed 36 h later and normalized to those of β-galactosidase. Transcriptional activity of E47 in each sample is presented relative to that in the absence of N1-IC with standard deviations from at least three independent experiments. For protein analysis, indicated amounts of E47 or E12 and N1-IC were cotransfected with a GFP expression vector. Whole lysates were probed with anti-E47, GFP and N1-IC antibodies. (B) A proteasome inhibitor blocks the degradation of E47 protein induced by N1-IC. Immunoblot assays were carried out with NIH3T3 cells transfected with 1 µg of E47 expressing plasmid with or without 4 µg of the N1-IC construct and treated with the indicated amounts of MG-132 proteasome inhibitor, leupeptin or dimethylsulfoxide vehicle for 3 h prior to harvest. C indicates loading control. (C) Activation of endogenous Notch receptors enhances degradation of E47. The NIH3T3 fibroblasts expressing Notch ligand, Jagged-1 (J) or not (C), were infected with a retroviral construct producing both E47 and GFP. Cells were harvested 3 days after infection. Whole-cell lysates were analyzed using immunoblotting (IB) with antibodies against E47 and GFP. Nuclear extracts were used for detecting N1-IC. Another aliquot of the infected cells was used to isolate RNA for RT–PCR assays of HES1 and GAPDH mRNA.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.