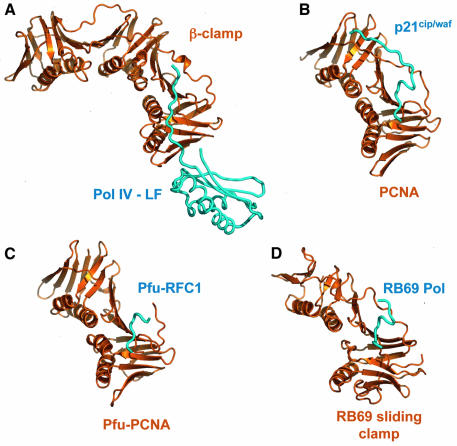
Fig. 5. Binding site conservation in processivity clamp interactions. (A) Secondary structure cartoon of the E.coli β-clamp–Pol IV-LF complex compared with other processivity clamp–peptide complexes. (B) Human PCNA–p21cip/waf peptide. (C) Pyrococcus furiosus PCNA–replication factor C peptide. (D) Phage RB69 sliding clamp–DNA polymerase. Despite the very low level of sequence similarity between the bacterial, mammalian, archaeal and viral clamps, the gross topology of the peptide-binding site is conserved in all the systems.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.