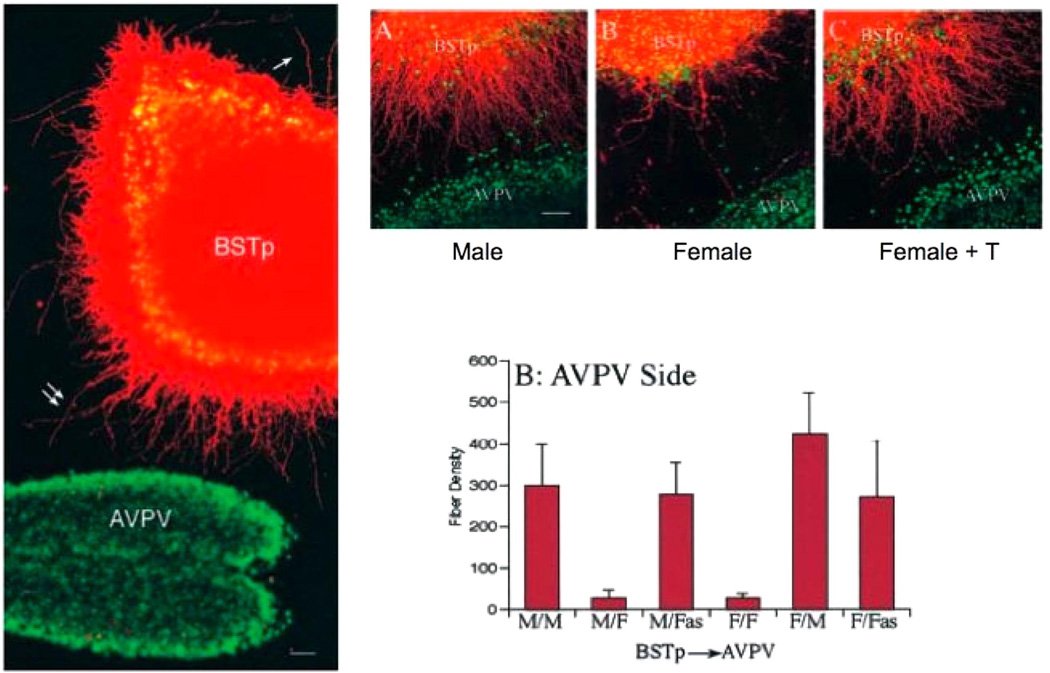
FIG 3.
Mechanisms of estradiol action for establishing a sexually dimorphic projection from the principle nucleus bed nucleus of the stria terminals (BSTp) to the anteroventral periventricular nucleus (AVPV). The AVPV is notable both for its central role in the control of the female-specific LH surge and for being larger in females than males. There is also a substantive sex difference in the size of the afferent projection from the BSTp to the AVPV, being up to 10-fold larger in males. To distinguish whether this sex difference in innervation arises in the BSTp or the AVPV, Simerly and colleagues (103)developed mixed-sex cocultures of the two nuclei. The BSTp is labeled with DiI (pseudo-colored red), and the AVPV is visualized with a Hoeschst stain (visualized here as green). Note the neuronal processes originating from the BSTp explant and extending toward the AVPV (left panel). The use of mixed sex cultures is illustrated with representative photomicrographs in the top panel and graphically by fiber density in the bottom panel. When a male-derived BSTp was paired with a male-derived AVPV (M/M), there were significantly more neurites extending toward the AVPV compared with a male-derived BSTp cocultured with a female-derived AVPV (M/F), and was not different from female-female cocultures (F/F). A female-derived BSTp innervated a male-derived AVPV (F/M) to the same degree as a M/M coculture, and treatment of females with testosterone before coculturing with a male BSTp resulted in a neurite growth rate identical to that of M/M cocultures. These data demonstrate that a hormonally determined target derived factor in the AVPV directs the innervation by the BSTp to produce a sexually dimorphic neural circuit. [From Ibanez et al. (103), copyright 2001 by Society for Neuroscience.]