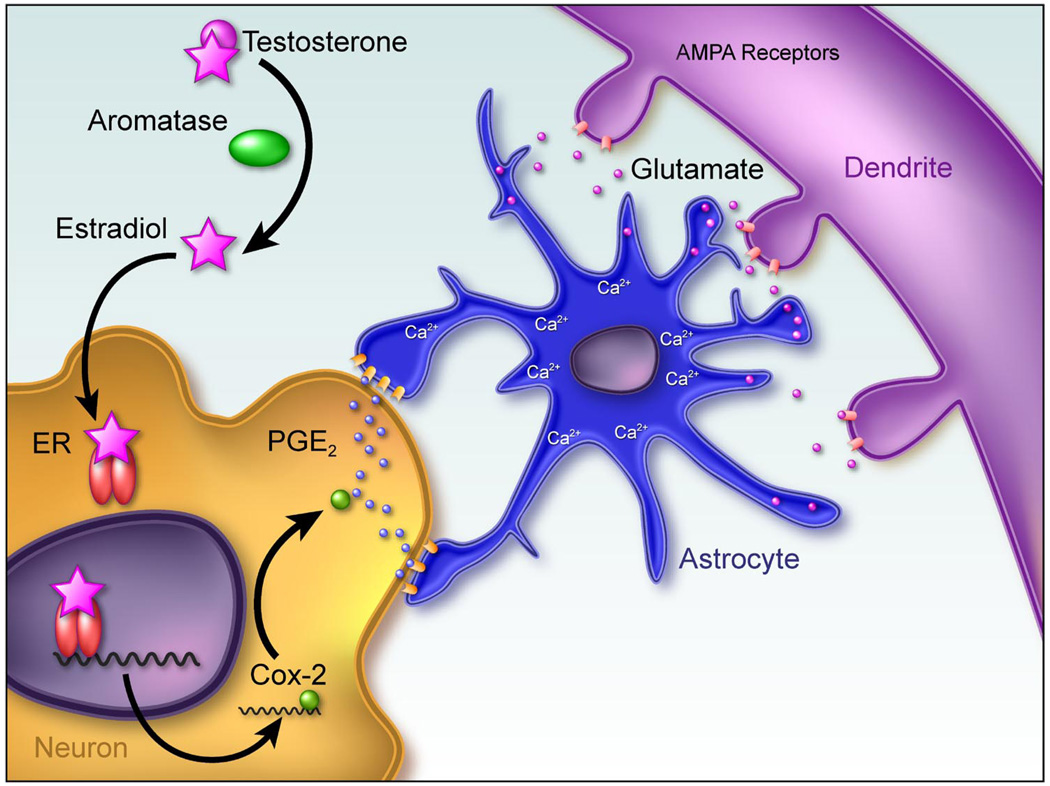
FIG 5.
Working model of mechanism of estradiol action establishing sexually dimorphic synaptic patterning in the preoptic area. The preoptic area (POA) is the critical brain region controlling expression of male sexual behavior and exhibits some of the most robust sex differences in the brain. In addition to the sexually dimorphic nucleus, male POA neurons have about twice as many dendritic spines as females, and this level of spines can be induced in females by treatment with estradiol during the perinatal sensitive period. Dendritic spines are the primary site for excitatory synapses. Astrocytes are also more complex in the male POA, with longer and more frequently branching processes. Both of these morphological sex differences are the result of estradiol action in the neonatal brain (4). The initiating event is the induction of COX-2, a pivotal enzyme in the production of prostanoids and specifically linked to an increased synthesis and release of prostaglandin E2 (PGE2). Receptors for PGE2 are G protein linked and can be found on astrocytes. Activation of EP receptors can induce glutamate release from astrocytes in a calcium-dependent manner, and glutamate induces the formation of dendritic spines. In this system, application of PGE2 induces a 2- to 3-fold increase in the density of dendritic spines on POA dendrites, and this effect can be blocked by antagonists to the glutamate AMPA receptor. Thus, in this model, a critical neuronal/astrocytic cross-talk is believed to be essential for PGE2 to induce a sexually dimorphic synaptic pattern determined by estradiol.