Abstract
Objectives
Cardiovascular mortality is increased in systemic lupus erythematosus (SLE). Increased plasma concentrations of N-terminal pro brain natriuretic peptide (NT-proBNP) are associated with cardiovascular morbidity and mortality in the general population. We examined the hypothesis that NT-proBNP concentrations are higher in patients with SLE, and are related to inflammation, augmentation index, coronary atherosclerosis, and cardiovascular risk factors.
Methods
Serum concentrations of NT-proBNP were measured in 113 patients with SLE and in 80 control subjects. Coronary calcification and augmentation index were measured by electron beam computed tomography and non-invasive pulse wave analysis, respectively.
Results
Patients with SLE had higher concentrations of NT-proBNP [median 38.6 (IQR 2.5–126.9) pg/mL] than controls [11.7 (1.6–47.9) pg/mL] (P=0.002). Augmentation index was higher in patients with SLE [25.0% (20.5%–31.5%)] than controls [20.5% (12.0%–29.0%)], (P=0.04). In patients with SLE, NT-proBNP concentrations were associated with disease damage (rho=0.31, P<0.001) and duration (rho=0.21, P=0.02) but not with disease activity, CRP, ESR, TNF-α, IL-6, coronary calcium score, or augmentation index (P all ≥0.18).
Conclusions
Patients with SLE have increased concentrations of NT-proBNP but this is not explained by atherosclerotic burden, augmentation index, or inflammatory state.
Keywords: systemic lupus erythematosus, atherosclerosis, NT-proBNP
INTRODUCTION
Several biomarkers are associated with cardiovascular risk in the general population. One such a biomarker is N-terminal pro brain natriuretic peptide (NT-proBNP), a hormone synthesized and secreted primarily in the heart in response to myocyte stretch.1 Measurement of plasma NT-proBNP concentrations is useful to diagnose and monitor heart failure and to determine its prognosis.2,3 However, more recent studies suggest that NT-proBNP is also associated with atherosclerosis. Data from the Framingham Heart Study indicate that higher concentrations of BNP are associated with increased the risk of cardiovascular events.4 This association between NT-proBNP and atherosclerosis was independent of heart failure in patients with diabetes,5 and in the general population.6
Patients with systemic lupus erythematosus (SLE) have accelerated atherosclerosis and increased cardiovascular risk.7–10 However, this increased risk is not accounted for by traditional risk factors.11,12 In a recent study we showed that despite a markedly increased prevalence of coronary atherosclerosis in patients with SLE, their Framingham cardiovascular risk scores were similar to those of matched controls.12 Thus, there is a need for identification of novel factors to better predict the development and presence of atherosclerosis in patients with SLE.
Little is known about NT-proBNP concentration in the setting of inflammatory diseases, particularly in patients with lupus. Thus, we examined the hypothesis that NT-proBNP concentrations are increased in patients with SLE and associated with inflammation and cardiovascular risk factors, including coronary calcification, a non-invasive measure of coronary atherosclerosis, and augmentation index, a non-invasive measure of vascular stiffness.
METHODS
Subjects
One hundred and thirteen eligible patients 18 years of age or older who met the classification criteria for SLE13 with disease duration of more than one year, and 80 age and sex-matched control subjects were studied. These subjects are part of ongoing studies of cardiovascular disease in SLE and the characteristics of the patients and methods used have been described in detail previously.9,12,14–17 Patients were recruited from practices of local rheumatologists, through a Lupus Foundation newsletter, and by advertisements. Control subjects were recruited from patients’ acquaintances, by local advertisement, and from a database of volunteers maintained by the General Clinical Research Center. The study was approved by the Institutional Review Board of Vanderbilt University Hospital and all subjects gave written informed consent. Subjects with a history of myocardial infarction or any coronary procedure, or with evidence of congestive heart failure (CHF) as determined from the medical history, were excluded from this study. Information was obtained using a structured interview, physical examination, laboratory tests, and review of medical records. In patients, disease activity was ascertained by the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI),18 and disease damage was determined by the SLICC/ACR Damage Index (SDI).19. Hypertension was defined as the use of antihypertensive agents or a systolic blood pressure of 140 mm Hg greater, or a diastolic blood pressure of 90 mm Hg or greater.
Laboratory Tests
Serum NT-proBNP concentrations were measured by multiplex enzyme-linked immunosorbent assay [ELISA (Linco Research/Millipore Corp., Billerica, MA)] according to the manufacturers instructions with inter and intraassay coefficients of variation of 8.4% and 8.3% respectively. Serum interleukin-6 (IL-6) and tumor necrosis factor alpha (TNF-α) concentrations were measured by ELISA (Linco Research/Millipore Corp., Billerica, MA). Other laboratory tests included a complete blood count, creatinine, fasting total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, triglycerides, lipoprotein(a) [Lp(a)], and homocysteine. The glomerular filtration rate was estimated with the modification of diet in renal disease (MDRD) study equation.20
Procedures
Subjects underwent chest computed tomography imaging with an Imatron C-150 scanner (GE/Imatron, South San Francisco, CA) as described in detail previously, all scans were interpreted by a single expert investigator (PR) who was unaware of the subjects’ clinical status.9 The extent of coronary-artery calcification was calculated as described by Agatston et al.21
Sixty patients and 42 controls also underwent non-invasive pulse wave analysis using the commercially available SphygmoCor system (AtCor Medical, Sydney, Au). After at least 10 minutes of supine rest, peripheral blood pressure was measured twice by an automated sphygmomanometer and augmentation index determined by applanation tonometry. The tonometer was held at the point of maximal pulsation and pressed lightly against the radial artery. Measurements were recorded after at least twelve consecutive beats and the quality of the waveforms confirmed by the program software. After these measurements were obtained, the software generated a corresponding central aortic pressure waveform.22 Because it is affected by heart rate, augmentation index was normalized to a heart rate of 75 beats per minute.
Statistical Methods
Demographic characteristics are presented as median and interquartile range for continuous variables, and as frequencies and percentages for categorical variables. Univariate analyses were performed to compare differences among patients and controls using Wilcoxon rank sum tests for continuous variables, and Pearson’s chi-squared tests for categorical variables. Spearman correlation coefficients were used to determine the association between NT-proBNP, clinical characteristics and laboratory tests. A linear regression model was used to examine if the association between SLE and NT-proBNP was independent of potential confounders. Logarithmically transformed NT-proBNP was the dependent variable, disease status (SLE or control) the independent variable, and age, sex, race, BMI, hypertension and augmentation index the covariates. The assumptions of the linear regression model were assessed using the skewness-kurtosis test to check the distribution of the residuals. Based on a post hoc calculation, assuming a mean NT-proBNP concentration of 43.7 pg/ml and a standard deviation of 83.9 in control subjects, this study that included 113 patients and 80 controls, has power to detect a difference of 35.6 pg/ml. All the analyses used a two-sided significance level of 5 percent and were performed with the use of STATA software (version 9.1).
RESULTS
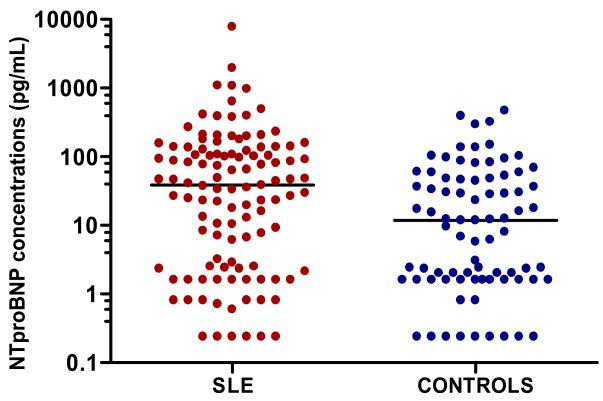
Demographic data for 113 patients with SLE and 80 control subjects are shown in Table 1. There was a predominance of white females in both patient and control groups and the two groups were of similar age. The mean duration of disease among patients with SLE was eight years, their median disease activity measured by the SLEDAI was 4 (0–6), and median damage, as quantified by the SLICC score, was 0 (0–1). Patients with SLE had higher NT-proBNP concentrations [38.6 (2.5–126.9) pg/mL] than controls [11.7 (1.6–47.9) pg/mL] (P=0.002). (Figure 1) The differences remained significant after adjustment for age, sex, race, hypertension, BMI, and augmentation index (p=0.026).
Table 1.
Characteristics of Patients with SLE and Control Subjects
| CHARACTERISTICS | Patients with SLE (n=113) | Control subjects (n=80) | P VALUE |
|---|---|---|---|
| Age (years) | 40 (31–47) | 41 (30–49) | 0.98 |
| Female (%) | 92% | 86% | 0.23 |
| White race (%) | 67% | 73% | 0.53 |
| Current Smoking (%) | 25% | 24% | 1.0 |
| Pack/years of smoking | 0 (0–5) | 0 (0–0.7) | 0.26 |
| Diabetes (%) | 4% | 3% | 1.0 |
| Hypertension (%) | 45% | 16% | <0.001 |
| Body Mass Index (kg/m2) | 27.3 (23.8–33.2) | 25.2 (22.1–30.0) | 0.04 |
| Family history of early CAD (%) | 19% | 11% | 0.16 |
| Total cholesterol (mg/dl) | 165 (141–204) | 180 (154–206) | 0.13 |
| Low-density lipoprotein (mg/dl) | 96 (78–129) | 108 (89–137) | 0.04 |
| High-density lipoprotein (mg/dl) | 48 (36–55) | 47 (38–61) | 0.58 |
| Homocysteine (μmol/L) | 9.2 (7.3–11.1) | 7.6 (6.5–8.9) | <0.001 |
| Augmentation index¶ (%) | 25 (21–32) | 21 (12–29) | 0.04 |
| White blood cells (per/μl) | 5,600 (4,300–7,700) | 6,000 (4,900–6,900) | 0.45 |
| Platelets (thou per/μl) | 244 (211–299) | 267 (232–312) | 0.07 |
| Creatinine (mg/dl) | 0.8 (0.7–0.9) | 0.8 (0.7–0.9) | 0.84 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | 95 (79–109) | 91 (82–104) | 0.80 |
| Triglycerides (mg/dl) | 103 (71–150) | 81 (62–108) | 0.03 |
| Lipoprotein (a) (mg/dl) | 12 (5–37) | 11 (5–32) | 0.71 |
| TNF-α (pg/ml) | 4.8 (3.1–7.9) | 2.4 (1.8–3.0) | <0.001 |
| IL-6 (pg/ml) | 5.7 (2.3–22.4) | 1.8 (0.9–4.7) | <0.001 |
| Coronary calcification (Agatston Units, mean±SD) | 43.4±189.8 | 3.8±27.9 | 0.002 |
Data available in 60 patients with SLE and 42 control subjects
Figure 1. Concentrations of amino-terminal brain natriuretic prepropeptide (NT-proBNP) in patients with SLE and control subjects.
Horizontal line represents the median. P=0.002 comparing SLE vs. controls.
Augmentation Index
Patients with SLE had a higher augmentation index [25.0% (20.5%–31.5%)] than controls [20.5% (12.0%–29.0%)], (P=0.04). Augmentation index was associated with diastolic blood pressure (rho=0.32, P=0.01), systolic blood pressure (rho=0.28, P=0.03), and Framingham score (rho=0.26, P=0.05), but not with disease activity (P=0.55) or disease duration (P=0.62). After adjusting for age, sex, race, and height the association between SLE and augmentation index remained significant (β=4.2, P=0.03).
NT-proBNP and cardiovascular risk factors
In patients with lupus, NT-proBNP concentrations were significantly higher in women 44.8 (6.8–134.6) pg/ml than men 2.3 (1.6–6.0) pg/mL, P=0.02); in controls this trend was less marked, 12.1 (2.0–47.9) pg/ml in women compared to 1.6 (1.6–58.6) pg/ml in men (P=0.46). In patients with SLE, neither coronary atherosclerosis, as determined by the Agatston coronary calcification score, nor traditional cardiovascular risk factors were associated with NT-proBNP concentrations. (Table 2)
Table 2.
Association between clinical variables and NT-proBNP concentrations in patients with SLE and control subjects
| Categorical Variables | Patients with SLE | Control subjects |
|---|---|---|
| Sex | ||
| Male | 2.3 (1.6–6.0) | 1.6 (1.6–58.6) |
| Female | 44.8 (6.8–134.6)* | 12.1 (2.0–47.9) |
| Race | ||
| Caucasian | 42.3 (3.0–130.7) | 16.3 (1.6–59.7) |
| Others | 35.1 (2.5–106.6) | 2.4 (1.6–16.0) |
| Continuous Variables | Spearman Rho | Spearman Rho |
| Age (years) | 0.08 | 0.04 |
| Body mass index | −0.20* | −0.14 |
| LDL Cholesterol (mg/dl) | −0.15 | 0.04 |
| Homocysteine (μmol/L) | 0.15 | −0.08 |
| Creatinine | 0.11 | 0.24 |
| Estimated glomerular filtration rate (ml/min/1.73 m2) | −0.19 | 0.04 |
| Pack years of smoking | −0.09 | 0.14 |
| Agatston score | 0.06 | −0.05 |
| Augmentation Index¶ | 0.14 | 0.08 |
| Systolic blood pressure (mmHg) | −0.02 | 0.08 |
| Diastolic blood pressure (mmHg) | −0.01 | −0.27* |
| Disease Duration (years) | 0.21* | NA |
| SLEDAI | 0.08 | NA |
| SLICC | 0.31** | NA |
| Corticosteroids cumulative dose units | −0.03 | NA |
| TNF-α pg/mL | 0.13 | 0.27* |
| IL-6 pg/mL | 0.13 | −0.05 |
| C-reactive protein pg/mL | −0.02 | NA |
| Erythrocyte sedimentation rate pg/mL | 0.05 | NA |
P=<0.05
P<0.001 For categorical variables, P values represent comparisons between male and female, or Caucasians and non-Caucasians.
NA: not applicable.
Data available in 60 patients with SLE and 42 control subjects
NT-proBNP and SLE disease markers
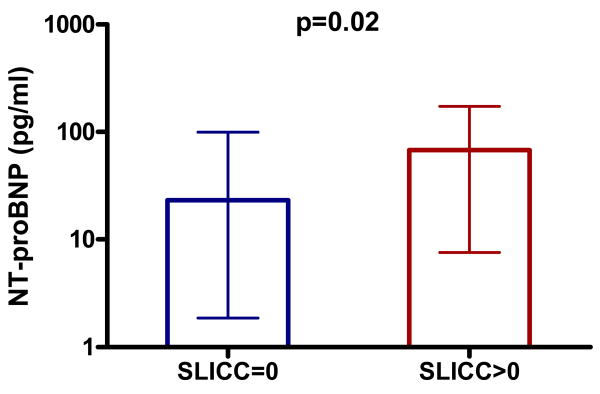
In patients with SLE, NT-proBNP concentrations were associated with the SLICC damage score (rho=0.31, P<0.001) and disease duration (rho=0.21, P=0.02), but not with disease activity (SLEDAI) (P=0.41) nor with markers of acute inflammation such as CRP (P=0.81), ESR (P=0.60) and TNF-α (P=0.18) and IL-6 (P=0.17) concentrations. (Table 2)
DISCUSSION
The main findings of this study are that patients with SLE have increased concentrations of NT-proBNP, and elevated concentrations are not associated with markers of vascular stiffness, coronary atherosclerosis, or acute inflammation. As described by Karadag, we found that patients with SLE had higher concentrations of NT-proBNP than control subjects.23 However, our study defined the association of NT-proBNP with coronary calcification, arterial stiffness and selected markers of inflammation in a larger group of patients.
NT-proBNP is a cardiac biomarker with clinical utility in the diagnosis and management congestive heart failure. In patients with heart failure, BNP is produced primarily by cardiac myocytes as a mechanism to offset left ventricular dysfunction.24–28 Another source of NT-proBNP may be the intima of human coronary arteries in response to ischemia.27,28 Studies in the general population suggest that higher concentrations of NT-proBNP, although not as high as those observed in heart failure, are a marker of coronary atherosclerosis.6 Interestingly, as we also observed in patients with SLE, NT-proBNP concentrations in the general population were higher in women than men,29 suggesting that the biomarker may be particularly useful in predicting atherosclerotic disease in women - a group in whom traditional risk factors such as Framingham score perform poorly.30,31
In addition to its association with coronary calcification in the general population, NT-proBNP is related to arterial stiffness. In patients with diabetes, high-normal concentrations of NT-proBNP were associated with augmentation index.32 That association was significant after statistical adjustment for modifiable cardiovascular risk factors, but not when additional adjustments for age and sex were performed. Our results show that patients with lupus have increased arterial stiffness as has been reported by others.33 however, the association between NT-proBNP and augmentation index was not significant.
We have previously reported that the prevalence and severity of coronary artery calcification is increased in this population of patients with SLE compared to age and sex-matched controls.9 However, neither coronary atherosclerosis (as measured by the presence and severity of calcification), nor augmentation index (as a marker of arterial stiffness), were associated with higher concentrations of NT-proBNP. There are several potential explanations for these findings. First, elevated NT-proBNP concentrations could precede the development of coronary atherosclerosis or increases in augmentation index, and thus, identify patients with clinically silent vascular disease who may be at increased risk of developing atherosclerosis subsequently. Second, there may be no association between atherosclerosis and elevated NT-proBNP concentrations in patients with SLE; these elevated concentrations could be due to other mechanisms, for example, asymptomatic myocardial dysfunction. Further studies will be required to examine these possibilities.
Findings in other populations have suggested that there may be a relationship between NT-proBNP and inflammation. For example, in patients with severe sepsis, NT-proBNP concentrations were comparable to those found in heart failure,34 and high concentrations were associated with increased mortality.35 Also, increased NT-proBNP concentrations were associated with higher concentrations of CRP in patients with chronic renal failure,36 and recently, we found that NT-proBNP concentrations were increased and associated with markers of acute inflammation in patients with rheumatoid arthritis.(unpublished data). However, in contrast to our findings in patients with rheumatoid arthritis, there was no association between NT-proBNP and CRP, ESR, TNF-α, or IL-6 in patients with lupus.
There was a statistically significant positive correlation between NT-proBNP and TNF-α in control subjects but not in patients with SLE, despite the fact that concentrations of TNF-α were significantly higher in patients with SLE. This suggests that the mechanisms increasing TNF-α in SLE are not correlated with those increasing NT-proBNP and may in fact act to obscure a relationship between baseline TNF-α and NT-proBNP.
We observed significantly higher concentrations of NT-proBNP in women than men. Although this is concordant with previous reports,37 men only accounted for 8% of patients with SLE and therefore the magnitude of this difference should be interpreted with caution.
Our findings should be interpreted in the light of the study design. First, echocardiographic studies were not performed; thus, we cannot exclude the possibility that subclinical myocardial dysfunction or hypertrophy were associated with increased concentrations of NT-proBNP. Second, renal clearance was not measured, and although there was no association between NT-proBNP with serum creatinine, the correlation between the estimated glomerular filtration rate and NT-proBNP concentrations was significant; thus, we cannot rule out subtle impairment of renal function as another potential confounder. Third, the majority of our patients had low to moderate lupus disease activity; nevertheless, it is in this population, free of confounding effects such as renal failure, that coronary calcification was increased.9 However, our findings may not necessarily apply to patients with more severe disease activity. Fourth, although the analysis was adjusted for potential differences related to hypertension and BMI, residual confounding due to additional unmeasured variables cannot be excluded. Also, the cross-sectional design does not provide a temporal sequence; therefore, longitudinal data to evaluate if concentrations of BNP provide prognostic information independent of other cardiovascular risk markers will be of interest.
In conclusion, patients with SLE have increased concentrations of NT-proBNP and this is not explained by atherosclerotic burden, augmentation index or current inflammatory state.
Figure 2. NTproBNP concentrations in patients with SLE with and without disease damage.
Error bars represent median and interquartile range.
P-value calculated using Wilcoxon rank sum test
Acknowledgments
We thank Mrs. Carol Brannon who assisted with patient recruitment and data entry.
This study was supported by grants (HL04012, HL65082 and GM5M01-RR00095) from the National Institutes of Health and by grants from the Lupus Foundation of America, Nashville Chapter, and the Lupus Clinical Trials Consortium. Dr. Avalos is funded in part by a grant from the American College of Rheumatology.
Reference List
- 1.Levin ER, Gardner DG, Samson WK. Natriuretic Peptides. N Engl J Med. 1998;339(5):321–328. doi: 10.1056/NEJM199807303390507. [DOI] [PubMed] [Google Scholar]
- 2.Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 3.Troughton RW, Frampton CM, Yandle TG, Espiner EA, Nicholls MG, Richards AM. Treatment of heart failure guided by plasma aminoterminal brain natriuretic peptide (N-BNP) concentrations. Lancet. 2000;355(9210):1126–1130. doi: 10.1016/s0140-6736(00)02060-2. [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 5.McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47(5):874–880. doi: 10.1161/01.HYP.0000216794.24161.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdullah SM, Khera A, Das SR, et al. Relation of Coronary Atherosclerosis Determined by Electron Beam Computed Tomography and Plasma Levels of N-terminal Pro-Brain Natriuretic Peptide in a Multiethnic Population-Based Sample (The Dallas Heart Study) The American Journal of Cardiology. 2005;96(9):1284–1289. doi: 10.1016/j.amjcard.2005.06.073. [DOI] [PubMed] [Google Scholar]
- 7.Manzi S, Meilahn EN, Rairie JE, et al. Age-specific incidence rates of myocardial infarction and angina in women with systemic lupus erythematosus: comparison with the Framingham Study. Am J Epidemiol. 1997;145(5):408–415. doi: 10.1093/oxfordjournals.aje.a009122. [DOI] [PubMed] [Google Scholar]
- 8.Urowitz MB, Gladman DD. Late mortality in SLE--“the price we pay for control”. J Rheumatol. 1980;7(3):412–416. [PubMed] [Google Scholar]
- 9.Asanuma Y, Oeser A, Shintani AK, et al. Premature coronary-artery atherosclerosis in systemic lupus erythematosus. N Eng J Med. 2003;349(25):2407–2415. doi: 10.1056/NEJMoa035611. [DOI] [PubMed] [Google Scholar]
- 10.Roman MJ, Shanker BA, Davis A, et al. Prevalence and correlates of accelerated atherosclerosis in systemic lupus erythematosus. N Eng J Med. 2003;349(25):2399–2406. doi: 10.1056/NEJMoa035471. [DOI] [PubMed] [Google Scholar]
- 11.Esdaile JM, Abrahamowicz M, Grodzicky T, et al. Traditional Framingham risk factors fail to fully account for accelerated atherosclerosis in systemic lupus erythematosus. Arthritis Rheum. 2001;44(10):2331–2337. doi: 10.1002/1529-0131(200110)44:10<2331::aid-art395>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 12.Chung CP, Oeser A, Avalos I, Raggi P, Stein CM. Cardiovascular risk scores and the presence of subclinical coronary artery atherosclerosis in women with systemic lupus erythematosus. Lupus. 2006;15(9):562–569. doi: 10.1177/0961203306071870. [DOI] [PubMed] [Google Scholar]
- 13.Tan EM, Cohen AS, Fries JF, et al. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25(11):1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 14.Asanuma Y, Chung CP, Oeser A, et al. Increased concentration of proatherogenic inflammatory cytokines in systemic lupus erythematosus: relationship to cardiovascular risk factors. J Rheumatol. 2006;33(3):539–545. [PubMed] [Google Scholar]
- 15.Oeser A, Chung CP, Asanuma Y, Avalos I, Stein CM. Obesity is an independent contributor to functional capacity and inflammation in systemic lupus erythematosus. Arthritis Rheum. 2005;52(11):3651–3659. doi: 10.1002/art.21400. [DOI] [PubMed] [Google Scholar]
- 16.Turner E, Dishy V, Chung CP, et al. Endothelial Function in Systemic Lupus Erythematosus: Relationship to Disease Activity, cardiovascular Risk Factors, Corticosteroid Therapy and Coronary Calcification. Vascular Health and Risk Management. 2005;1(4):357–360. doi: 10.2147/vhrm.2005.1.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chung CP, Avalos I, Oeser A, et al. High Frequency of the Metabolic Syndrome in Patients with Systemic Lupus Erythematosus: Association with Disease Characteristics and Cardiovascular Risk Factors. Ann Rheum Dis. 2007;66(2):208–214. doi: 10.1136/ard.2006.054973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630–640. doi: 10.1002/art.1780350606. [DOI] [PubMed] [Google Scholar]
- 19.Gladman D, Ginzler E, Goldsmith C, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363–369. doi: 10.1002/art.1780390303. [DOI] [PubMed] [Google Scholar]
- 20.Levey AS, Greene T, Kusek J, Beck GA. A Simplified Equation to Predict Glomerular Filtration Rate from Serum Creatinine. Journal of the American Society of Nephrology. 2000;11:155A. Ref Type: Abstract. [Google Scholar]
- 21.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Vaimonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15(4):827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson IB, Fuchs SA, Jansen IM, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16(12 Pt 2):2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 23.Karadag O, Calguneri M, Yavuz B, et al. B-type natriuretic peptide (BNP) levels in female systemic lupus erythematosus patients: what is the clinical significance? Clinical Rheumatology. 2007;26(10):1701–1704. doi: 10.1007/s10067-007-0575-4. [DOI] [PubMed] [Google Scholar]
- 24.Magga J, Marttila M, Mantymaa P, Vuolteenaho O, Ruskoaho H. Brain natriuretic peptide in plasma, atria, and ventricles of vasopressin- and phenylephrine-infused conscious rats. Endocrinology. 1994;134(6):2505–2515. doi: 10.1210/endo.134.6.8194476. [DOI] [PubMed] [Google Scholar]
- 25.Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5 Suppl):S81–S83. doi: 10.1016/j.cardfail.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Bruneau BG, Piazza LA, de Bold AJ. BNP gene expression is specifically modulated by stretch and ET-1 in a new model of isolated rat atria. Am J Physiol. 1997;273(6 Pt 2):H2678–H2686. doi: 10.1152/ajpheart.1997.273.6.H2678. [DOI] [PubMed] [Google Scholar]
- 27.Goetze JP, Christoffersen C, Perko M, et al. Increased cardiac BNP expression associated with myocardial ischemia. FASEB J. 2003;17(9):1105–1107. doi: 10.1096/fj.02-0796fje. [DOI] [PubMed] [Google Scholar]
- 28.Casco VH, Veinot JP, Kuroski de Bold ML, Masters RG, Stevenson MM, de Bold AJ. Natriuretic peptide system gene expression in human coronary arteries. J Histochem Cytochem. 2002;50(6):799–809. doi: 10.1177/002215540205000606. [DOI] [PubMed] [Google Scholar]
- 29.Wang TJ, Larson MG, Levy D, et al. Impact of age and sex on plasma natriuretic peptide levels in healthy adults. The American Journal of Cardiology. 2002;90(3):254–258. doi: 10.1016/s0002-9149(02)02464-5. [DOI] [PubMed] [Google Scholar]
- 30.Michos ED, Vasamreddy CR, Becker DM, et al. Women with a low Framingham risk score and a family history of premature coronary heart disease have a high prevalence of subclinical coronary atherosclerosis. Am Heart J. 2005;150(6):1276–1281. doi: 10.1016/j.ahj.2005.02.037. [DOI] [PubMed] [Google Scholar]
- 31.Michos ED, Nasir K, Braunstein JB, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184(1):201–206. doi: 10.1016/j.atherosclerosis.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 32.Dawson A, Davies JI, Morris AD, Struthers AD. B-Type Natriuretic Peptide Is Associated With Both Augmentation Index and Left Ventricular Mass in Diabetic Patients Without Heart Failure. American Journal of Hypertension. 2005;18(12):1586–1591. doi: 10.1016/j.amjhyper.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Roman MJ, Devereux RB, Schwartz JE, et al. Arterial Stiffness in Chronic Inflammatory Diseases. Hypertension. 2005;46(1):194–199. doi: 10.1161/01.HYP.0000168055.89955.db. [DOI] [PubMed] [Google Scholar]
- 34.Rudiger A, Gasser S, Fischler M, Hornemann T, von EA, Maggiorini M. Comparable increase of B-type natriuretic peptide and amino-terminal pro-B-type natriuretic peptide levels in patients with severe sepsis, septic shock, and acute heart failure. Crit Care Med. 2006;34(8):2140–2144. doi: 10.1097/01.CCM.0000229144.97624.90. [DOI] [PubMed] [Google Scholar]
- 35.Brueckmann M, Huhle G, Lang S, et al. Prognostic Value of Plasma N-Terminal Pro-Brain Natriuretic Peptide in Patients With Severe Sepsis. Circulation. 2005;112(4):527–534. doi: 10.1161/CIRCULATIONAHA.104.472050. [DOI] [PubMed] [Google Scholar]
- 36.Ortega O, Gallar P, Munoz M, et al. Association between C-reactive protein levels and N-terminal pro-B-type natriuretic peptide in pre-dialysis patients. Nephron Clin Pract. 2004;97(4):c125–c130. doi: 10.1159/000079170. [DOI] [PubMed] [Google Scholar]
- 37.Raymond I, Groenning BA, Hildebrandt PR, et al. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart. 2003;89(7):745–751. doi: 10.1136/heart.89.7.745. [DOI] [PMC free article] [PubMed] [Google Scholar]