Abstract
Context
Familial glucocorticoid deficiency (FGD) is a rare autosomal recessive disease, characterised by isolated glucocorticoid deficiency in the absence of mineralocorticoid deficiency. Inactivating mutations in the ACTH receptor (melanocortin-2-receptor, MC2R) are well described and account for ∼25% of cases. By contrast, activating MC2R mutations are extremely rare.
Patient
We report a child of Saudi Arabian origin who was diagnosed with FGD following hypoglycaemic episodes that resulted in spastic quadriplegia.
Methods and results
MC2R gene analysis revealed an unusual combination of two homozygous missense mutations, consisting of the novel mutation Y129C and the previously described F278C activating mutation. Parents were heterozygous at both of these sites. In vitro analysis of the Y129C mutation using a fluorescent cell surface assay showed that this mutant was unable to reach the cell surface in CHO cells stably transfected with MC2R accessory protein (MRAP), despite the demonstration of an interaction with MRAP by co-immunoprecipitation. The double mutant Y129C-F278C also failed to traffic to the cell surface.
Conclusion
The tyrosine residue at position 129 in the second intracellular loop is critical in MC2R folding and/or trafficking to the cell surface. Furthermore, the absence of cell surface expression of MC2R would account for the lack of activation of the receptor due to the F278C mutation located at the C-terminal tail. We provide a novel molecular explanation for a child with two opposing mutations causing severe FGD.
Introduction
Familial glucocorticoid deficiency (FGD) (OMIM 202200) is a rare autosomal recessive condition clinically characterised by isolated glucocorticoid deficiency in the presence of normal mineralocorticoid production (1, 2). Affected individuals usually present during the neonatal period or by early childhood with symptoms resulting from either hypocortisolaemia or elevated plasma ACTH levels. Symptoms include hypoglycaemic seizures, hyperpigmenation, recurrent infections, failure to thrive, collapse and coma (2). Severe long term neurological consequences have been described, which may reflect the severity and number of hypoglycaemic episodes during childhood. Severe loss-of-function ACTH receptor/melanocortin-2-receptor (MC2R) mutations have, however, been associated with mild derangements in the renin–angiotensin–aldosterone axis (3).
Inactivating mutations of the MC2R as a cause of FGD were first described in 1993 (4, 5). Many more inactivating mutations were subsequently found, accounting for ∼25% of FGD cases. The majority of these are summarised in (2). A further 15–20% of FGD cases are due to mutations in MC2R accessory protein (MRAP), which is required for the trafficking and cell surface expression of the MC2R (6). More recently, a patient with FGD was reported to have compound heterozygosity of a frameshift mutation in the coding region and a single-base substitution in the promoter of the ACTH receptor (7).
MC2R, the second of the melanocortin receptor family to be cloned (8), is highly conserved across species (9) and is distinct from the other four melanocortin receptors, responding only to its specific ligand, ACTH. Several groups have hypothesised that the MC2R could be involved in ACTH-independent causes of cortisol excess such as adrenal hyperplasia or neoplastic conditions. Studies on adrenocortical adenomas and neoplasias failed to identify mutations in the MC2R (10, 11). However, there is evidence that the MC2R mRNA expression is increased in aldosterone and cortisol secreting adenomas (12, 13) and loss of heterozygosity of the MC2R locus has been demonstrated in adrenocortical tumours (14).
The first and so far only activating homozygous germ-line MC2R mutation causing glucocorticoid excess was found in a patient with cyclical Cushing's syndrome (15). The missense mutation, resulting in the substitution of a phenylalanine residue by a cysteine at position 278 (F278C) in the C-terminal tail, was functionally characterised by Swords et al. (15).
A combination of cAMP accumulation, ACTH competitive binding and receptor internalisation assays were used to show that the F278C mutant receptor was at the cell surface and exhibited increased basal activity behaving as a receptor with constitutive activity. The constitutive activity of the mutant receptor was thought to result from impaired receptor desensitisation (15).
A second cause of increased receptor basal activity was described in a patient with two mutations (C21R and S247G) on the same allele of the MC2R that had synergistic activity resulting in a syndrome of ACTH hypersensitivity (16, 17).
Subject and methods
Patient report
The index case is the youngest of three children born to first cousins of Saudi Arabian origin. Born at term with normal birth weight, he presented with hypoglycaemia, apnoea and cyanosis within the first 24 hours of life. He was diagnosed with pneumonia and sepsis and treated with antibiotics. At 3 months of age, he presented with recurrent cough and shortness of breath, and was treated for a chest infection. He subsequently presented with collapse, bradycardia and hypotension at 8 months of age, requiring resuscitation and admission to intensive care. He was found to be hypoglycaemic and developed seizures. Investigations revealed a low serum cortisol and grossly elevated plasma ACTH level (>1250 pg/ml; N.R. 10–46). Serum electrolytes and aldosterone levels were normal. He was diagnosed with adrenal insufficiency and initiated on hydrocortisone (HC) replacement. He suffered further seizures and developed spastic quadriplegia, visual impairment and neurosensory deafness. An older sibling died at the age of 7 months after developing seizures post-vaccinations. She was described as having a dark complexion similar to the index child. At 1.3 years of age the index child was reassessed while on HC. His height and weight were between 10 and 25th percentile, while his head circumference was on the 0.4th centile. He was noted to be darkly pigmented despite replacement with HC dosage of 20 mg/m2 per day, a common feature of FGD (2). This correlated to an extremely high plasma ACTH level of 1169 ng/l (N.R. 0–50), aldosterone and renin levels were normal (aldosterone 251 pmol/l (N.R. recumbent <440); plasma renin activity 38.9 ng/l (recumbent 43–61)). Serum electrolytes and glucose was normal. A diagnosis of FGD was suspected and a molecular diagnosis was sought.
Mutational analysis of MC2R
Genomic DNA was extracted from blood leukocytes. PCR of the single coding exon was performed using specific intronic primers (F, 5′-AGTCCAAGTAACATCCCC-3′, R, 5′-CCATGGATTCTAAAACCAGGG-3′). After the initial denaturing step at 95 °C for 5 min, a touchdown program was used i.e. 16 cycles of 95 °C for 30 s, x °C for 30 s and 72 °C for 1 min (where x was 65 °C for the first cycle which then decreased by 1 °C with each cycle). This was followed by 25 cycles of 95 °C for 30 s, 45 °C for 30 s and 72 °C for 1 min, with a final extension step at 72 °C for 5 min. PCR products were sequenced using the ABI Prism Big Dye Sequencing kit and an ABI 377 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA) in accordance with the manufacturer's instructions.
MC2R expression vectors and MRAP stable cell line generation
The human MC2R triple HA tagged (3xHA-MC2R) cDNA clone was obtained from the Missouri S&T cDNA Resource Center (www.cdna.org). 3xHA-Y129C and 3xHA-F278C single MC2R mutants, in addition to 3xHA-Y129C-F278C double mutant MC2R were generated using the Quick Change site-directed mutagenesis protocol (Stratagene, Amsterdam, The Netherlands). Oligonucleotide primers are available on request. Mutagenesis was confirmed by DNA sequencing. The full length human MRAPα cDNA was cloned into pcDNA3.1 (Invitrogen). CHO cells were grown in DMEM/F12 (1:1 ratio) with 10% FCS and 1% penicillin/streptomycin mix. Cells were transfected with MRAPα construct using Lipofectamine 200 (Invitrogen) and selected in 700 μg/ml Genetecin (G418) (Life Sciences, Paisley, UK). Colonies were selected after serial dilutions. Selected clones were screened by RT-PCR to ensure adequate expression of MRAPα mRNA. Transient transfection of wild-type (WT) 3xHA-MC2R was performed to ensure surface expression of the receptor. Cells stably transfected with pcDNA3.1 alone were used as a negative control.
Western blot and co-immunoprecipitation (Co-IP)
The interaction between MRAP and mutant MC2R was assessed by Co-IP using a FLAG-tagged MRAP construct and 3xHA-Y129C mutant receptor. Cells were transiently transfected with the vector(s), MRAP-FLAG, WT 3xHA-MC2R alone or in combination as well as MRAP-FLAG combined with mutant 3xHA-Y129C receptor. For 24 h post-transfection, cells were scraped into lysis buffer (1% DM in PBS, with protease inhibitor cocktail 1:1000) and incubated on ice for 30 min before centrifugation at 19 000 g for 30 min at 4 °C. Overnight immunoprecipitation was performed using anti-HA agarose (Sigma–Aldrich Corp). Western blotting was performed using anti-FLAG antibodies (Sigma–Aldrich Corp.) at a dilution of 1 in 2000.
Fluorescent cell surface assay and confocal microscopy
MRAPα stable cells were grown in 24-well plates and transfected with WT 3xHA-MC2R, 3xHA-Y129C, 3xHA-F278C or 3xHA-Y129C-F278C construct. For 24 h post-transfection; cells were washed and fixed in 3.7% paraformaldehyde (10 min). Duplicate transfections were performed on a separate plate. Cells from one plate were permeabilised with PBS/0.05% Triton-X100. Both plates were then washed in PBS, blocked in the LI-COR Odyssey Blocking Buffer for 1 h, and exposed to primary anti-HA antibody (Sigma–Aldrich Corp.) for 1 h. Antibody dilution of 1/1000 was used to detect 3xHA-MC2R surface expression in non-permeabilised cells and total MC2R expression in permeabilised cells. After 3 washes in PBS, incubation with the secondary antibody (IRDye 800CW Goat Anti-Mouse IgG; LI-COR, Lincoln, NE, USA) was undertaken at a concentration of 1/1000 for 1 h. Three further PBS washes followed and the total fluorescence from each well was measured using the Odyssey Imaging System (LI-COR). Confocal microscopy was performed as described in (6).
Statistical analysis
Experiments were performed in duplicate, and the results represent the mean of three independent experiments (with the exception of 3xHA-F278C; n=2). Statistical significance was determined using a non-paired two-tailed Student's t-test.
Results
MC2R mutations
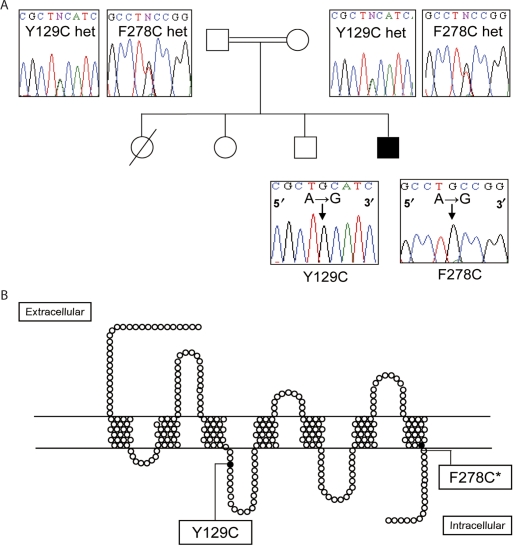
DNA analysis of the index child revealed two homozygous missense mutations. The first of these is a novel homozygous mutation of an A to G nucleotide substitution resulting in the Y129C change (substitution of the tyrosine residue with a cysteine at position 129). The second mutation, also A to G, is the previously described F278C activating mutation (substituting the phenylalanine at position 278 with a cysteine residue). Both these mutations were found in the homozygous state in the index child (Fig. 1) and in heterozygosity in both parents. In order for the child to inherit both mutations in a homozygous state, the two mutations in the parents must be located on the same allele.
Figure 1.
(A) Family tree and mutational analysis of the MC2R. Partial sequence of the MC2R showing the Y129C mutation and F278C mutation in heterozygosity in the parents and homozygosity in index case (filled box). (B) Pseudostructural plot of the MC2R showing the location of Y129C in the second intracellular loop and F278C* in the C-terminal tail. Full colour version of this figure available via http://dx.doi.org/10.1530/EJE-08-0636.
Y129C single mutant fails to traffic to the cell surface and is intracellularly retained, despite interacting with MRAP: Y129C-F278C double mutant also fails to traffic to the cell surface
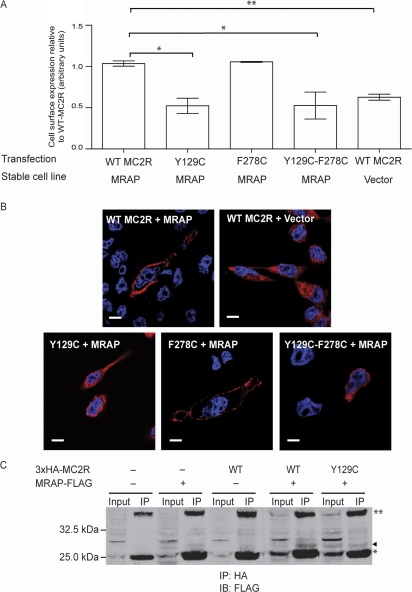
The transient transfection of WT 3xHA-MC2R into MRAPα stably transfected CHO cells enable efficient surface expression of the receptor in this heterologous cell line. The 3xHA-F278C mutant receptor traffics efficiently to the cell surface. By contrast, the 3xHA-Y129C and 3xHA-Y129C-F278C mutants fail to traffic to the plasma membrane in the presence of MRAP (Fig. 2). Confocal imaging shows surface localisation of the F278C mutant receptor but intracellular retention of the Y129C and Y129C-F278C double mutant protein. The images of the intracellular distribution of these retained receptors are comparable with those of the WT 3xHA-MC2R in the absence of MRAP. To assess whether the substitution of the tyrosine residue at position 129 affects the interaction with MRAP, a Co-IP technique was employed. Using CHO cells transiently co-transfected with 3xHA-Y129C and MRAP-FLAG, immunoprecipitation of mutant Y129C using anti-HA, resulted in Co-IP of MRAP-FLAG (Fig. 2A), detected by western blotting with an anti-FLAG antibody. This demonstrated that the ability of the mutant to interact with MRAP is unaffected by the substitution of the tyrosine residue. The Y129C trafficking was one of the mutations studied as part of a larger study (21).
Figure 2.
(A) Failed surface localisation of the Y129C single mutant receptor and Y129C-F278C double mutant receptor in MRAP expressing cell lines. In comparison, WT MC2R and F278C mutation traffics efficiently to the plasma membrane in the presence of MRAP. Fluorescent cell surface assay performed in duplicate in three independent experiments (with the exception of F278C experiments, n=2). Values on y-axis represent fluorescent count normalised for total cell number. Error bars represent s.e.m. *P<0.05, **P<0.01. Surface expression of Y129C was included as part of a larger study (21) (B) Confocal images to show the intracellular retention of the 3xHA-Y129C and Y129C-F278C mutant in MRAP stable cell lines. This is similar to WT 3xHA-MC2R retention seen in the absence of MRAP. By contrast, the WT 3xHA-MC2R and 3xHA-F278C in the presence of MRAP localises to the cell surface efficiently. Size bar represents 10 μm. (C) Co-immunoprecipitation of MRAP-FLAG and HA tagged receptor show direct interaction between MRAP and Y129C receptor as well as WT MC2R. MRAP – FLAG band indicated by (◂). IP, immunoprecipitation; IB, immunoblotting, *light chain, **heavy chain. Full colour version of this figure available via http://dx.doi.org/10.1530/EJE-08-0636.
Discussion
The clinical case presented in this report is unique in that the DNA analysis confirms the presence of two MC2R mutations that have opposite effects. One of these mutations (F278C mutation) was previously studied in great detail and shown to cause constitutive MC2R activity. Specifically, surface expression of F278C mutant receptor was demonstrated by [125] ACTH binding assays (15). This was confirmed in our present study by immunofluorescent cell surface assay and confocal microscopy.
We therefore assessed the second mutation, a novel inactivating missense mutation, Y129C, located in the second intracellular loop in isolation and with the F278C mutation. We hypothesised that the Y129C mutation may affect cell surface expression of MC2R, which would provide an explanation for the apparent lack of activation secondary to the F278C mutation. We tested this hypothesis by studying the ability of the Y129C receptor to traffic to the cell surface, using a fluorescent cell surface assay. Transfection of the Y129C single and double mutant MC2R into CHO cells stably transfected with MRAP revealed that the Y129C mutant fails to reach the cell surface, despite its interaction with MRAP.
The tyrosine residue at position 129 lies in the DRY motif of the second intracellular loop. This motif is highly conserved in the entire rhodopsin subclass of G-protein coupled receptors (18, 19). Our data suggests that Y129 plays a vital role in folding or cell-surface trafficking of the receptor. Interestingly, functional analysis of the neighbouring amino acid R128C showed that this mutant traffics to the cell surface but fails to signal through loss of high-affinity binding (20, 21).
The index case was severely affected with spastic quadriplegia. Clinical severity may not always correlate with genotype. In the MC2R knockout mouse model, there is great variability in presentation, despite identical genotype and genetic background some mice died soon after birth while others were able to survive into adulthood (22).
In conclusion, we describe a child with two functionally opposing MC2R mutations. The inability of the Y129C mutant receptor to localise to the cell surface in this child essentially inactivates the ability of the F278C mutation to cause constitutive activity and a Cushing's syndrome phenotype, thus providing a unique molecular explanation for the existence of two opposing MC2R mutations resulting in FGD.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
L F C and T-T C are supported by M R C Clinical Research Training Fellowships (grant numbers G0600408, G0700581) and L A M by the Wellcome Trust (grant number 076430/Z/05/7).
Footnotes
(L F Chan and T-T Chung contributed equally to this work)
References
- Clark AJ, Metherell LA, Cheetham ME, Huebner A. Inherited ACTH insensitivity illuminates the mechanisms of ACTH action. Trends in Endocrinology and Metabolism. 2005;10:451–457. doi: 10.1016/j.tem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Chan LF, Clark AJ, Metherell LA. Familial glucocorticoid deficiency: advances in the molecular understanding of ACTH action. Hormone Research. 2008;2:75–82. doi: 10.1159/000111810. [DOI] [PubMed] [Google Scholar]
- Lin L, Hindmarsh PC, Metherell LA, Alzyoud M, Al Ali M, Brain CE, Clark AJ, Dattani MT, Achermann JC. Severe loss-of-function mutations in the adrenocorticotropin receptor (ACTHR, MC2R) can be found in patients diagnosed with salt-losing adrenal hypoplasia. Clinical Endocrinology. 2007;2:205–210. doi: 10.1111/j.1365-2265.2006.02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AJ, McLoughlin L, Grossman A. Familial glucocorticoid deficiency associated with point mutation in the adrenocorticotropin receptor. Lancet. 1993;8843:461–462. doi: 10.1016/0140-6736(93)90208-x. [DOI] [PubMed] [Google Scholar]
- Tsigos C, Arai K, Hung W, Chrousos GP. Hereditary isolated glucocorticoid deficiency is associated with abnormalities of the adrenocorticotropin receptor gene. Journal of Clinical Investigation. 1993;5:2458–2461. doi: 10.1172/JCI116853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metherell LA, Chapple JP, Cooray S, David A, Becker C, Ruschendorf F, Naville D, Begeot M, Khoo B, Nurnberg P, Huebner A, Cheetham ME, Clark AJ. Mutations in MRAP, encoding a new interacting partner of the ACTH receptor, cause familial glucocorticoid deficiency type 2. Nature Genetics. 2005;2:166–170. doi: 10.1038/ng1501. [DOI] [PubMed] [Google Scholar]
- Tsiotra PC, Koukourava A, Kaltezioti V, Geffner ME, Naville D, Begeot M, Raptis SA, Tsigos C. Compound heterozygosity of a frameshift mutation in the coding region and a single base substitution in the promoter of the ACTH receptor gene in a family with isolated glucocorticoid deficiency. Journal of Pediatric Endocrinology and Metabolism. 2006;9:1157–1166. doi: 10.1515/jpem.2006.19.9.1157. [DOI] [PubMed] [Google Scholar]
- Mountjoy KG, Robbins LS, Mortrud MT, Cone RD. The cloning of a family of genes that encode the melanocortin receptors. Science. 1992;5074:1248–1251. doi: 10.1126/science.1325670. [DOI] [PubMed] [Google Scholar]
- Logan DW, Bryson-Richardson RJ, Pagan KE, Taylor MS, Currie PD, Jackson IJ. The structure and evolution of the melanocortin and MCH receptors in fish and mammals. Genomics. 2003;2:184–191. doi: 10.1016/s0888-7543(02)00037-x. [DOI] [PubMed] [Google Scholar]
- Light K, Jenkins PJ, Weber A, Perrett C, Grossman A, Pistorello M, Asa SL, Clayton RN, Clark AJ. Are activating mutations of the adrenocorticotropin receptor involved in adrenal cortical neoplasia? Life Science. 1995;18:1523–1527. doi: 10.1016/0024-3205(95)00114-l. [DOI] [PubMed] [Google Scholar]
- Latronico AC, Reincke M, Mendonca BB, Arai K, Mora P, Allolio B, Wajchenberg BL, Chrousos GP, Tsigos C. No evidence for oncogenic mutations in the adrenocorticotropin receptor gene in human adrenocortical neoplasms. Journal Clinical Endocrinology and Metabolism. 1995;3:875–877. doi: 10.1210/jcem.80.3.7883845. [DOI] [PubMed] [Google Scholar]
- Reincke M, Beuschlein F, Lalli E, Arlt W, Vay S, Sassone-Corsi P, Allolio B. DAX-1 expression in human adrenocortical neoplasms: implications for steroidogenesis. Journal Clinical Endocrinology and Metabolism. 1998;7:2597–2600. doi: 10.1210/jcem.83.7.5095. [DOI] [PubMed] [Google Scholar]
- Arnaldi G, Mancini V, Costantini C, Giovagnetti M, Petrelli M, Masini A, Bertagna X, Mantero F. ACTH receptor mRNA in human adrenocortical tumors: overexpression in aldosteronomas. Endocrine Research. 1998;3–4:845–849. doi: 10.3109/07435809809032695. [DOI] [PubMed] [Google Scholar]
- Reincke M, Mora P, Beuschlein F, Arlt W, Chrousos GP, Allolio B. Deletion of the adrenocorticotropin receptor gene in human adrenocortical tumors: implications for tumorigenesis. Journal Clinical Endocrinology and Metabolism. 1997;9:3054–3058. doi: 10.1210/jcem.82.9.4211. [DOI] [PubMed] [Google Scholar]
- Swords FM, Baig A, Malchoff DM, Malchoff CD, Thorner MO, King PJ, Hunyady L, Clark AJ. Impaired desensitization of a mutant adrenocorticotropin receptor associated with apparent constitutive activity. Molecular Endocrinology. 2002;12:2746–2753. doi: 10.1210/me.2002-0099. [DOI] [PubMed] [Google Scholar]
- Hiroi N, Yakushiji F, Shimojo M, Watanabe S, Sugano S, Yamaguchi N, Miyachi Y. Human ACTH hypersensitivity syndrome associated with abnormalities of the ACTH receptor gene. Clinical Endocrinology. 1998;2:129–134. doi: 10.1046/j.1365-2265.1998.3971187.x. [DOI] [PubMed] [Google Scholar]
- Swords FM, Noon LA, King PJ, Clark AJ. Constitutive activation of the human ACTH receptor resulting from a synergistic interaction between two naturally occurring missense mutations in the MC2R gene. Molecular Cell Endocrinology. 2004;2:149–154. doi: 10.1016/j.mce.2003.10.052. [DOI] [PubMed] [Google Scholar]
- Savarese TM, Fraser CM. In vitro mutagenesis and the search for structure–function relationships among G protein-coupled receptors. Biochemistry Journal. 1992:1–19. doi: 10.1042/bj2830001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovati GE, Capra V, Neubig RR. The highly conserved DRY motif of class A G protein-coupled receptors: beyond the ground state. Molecular Pharmacology. 2007;4:959–964. doi: 10.1124/mol.106.029470. [DOI] [PubMed] [Google Scholar]
- Elias LL, Huebner A, Pullinger GD, Mirtella A, Clark AJ. Functional characterization of naturally occurring mutations of the human adrenocorticotropin receptor: poor correlation of phenotype and genotype. Journal Clinical Endocrinology and Metabolism. 1999;8:2766–2770. doi: 10.1210/jcem.84.8.5924. [DOI] [PubMed] [Google Scholar]
- Chung TT, Webb TR, Chan LF, Cooray SN, Metherell LA, King PJ, Chapple JP, Clark AJ. The majority of adrenocorticotropin receptor (melanocortin 2 receptor) mutations found in familial glucocorticoid deficiency type 1 lead to defective trafficking of the receptor to the cell surface. Journal Clinical Endocrinology and Metabolism. 2008;12:4948–4954. doi: 10.1210/jc.2008-1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida D, Nakagawa S, Nagai S, Sagara H, Katsumata H, Imaki T, Suzuki H, Mitani F, Ogishima T, Shimizu C, Kotaki H, Kakuta S, Sudo K, Koike T, Kubo M, Iwakura Y. Melanocortin 2 receptor is required for adrenal gland development, steroidogenesis, and neonatal gluconeogenesis. PNAS. 2007;46:18205–18210. doi: 10.1073/pnas.0706953104. [DOI] [PMC free article] [PubMed] [Google Scholar]