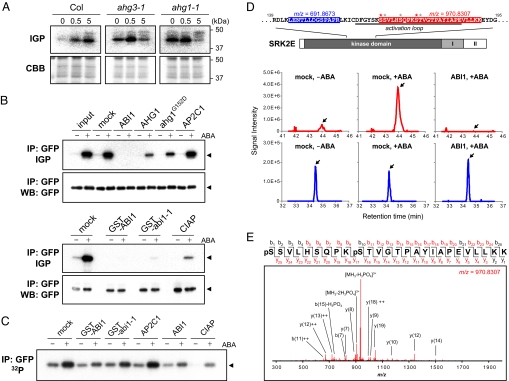
Fig. 3.
PP2C directly regulates SnRK2. (A) ABA-activated protein kinase activity in imbibed seeds of Col, ahg1–1, and ahg3–1 plants treated with 25 μM ABA for 0, 0.5, or 5.0 h. Upper panels show in-gel phosphorylation assay (IGP) using histone as a substrate. Lower panels are CBB-stained gel images. (B) Inactivation assay for SnRK2 by in vitro reaction with PP2Cs. SRK2E-GFP proteins were prepared by immunoprecipitation (IP) against an anti-GFP antibody from Arabidopsis cultured cells with or without ABA treatment. After incubation with recombinant PP2C proteins as indicated, SRK2E activity was monitored with an in-gel phosphorylation assay (IGP) using histone as a substrate. Immunoprecipitates were analyzed via Western blotting (WB) using an anti-GFP antibody. Arrows indicate SRK2E-GFP. (C) Dephosphorylation of 32P-labeled SRK2E-GFP by PP2Cs. Immunoprecipitated SRK2E-GFP was prepared from untreated or ABA-treated cultured cells labeled with 32P-phosphate, and incubated with PP2Cs as indicated. The phosphorylation level of SRK2E-GFP was monitored by autoradiography. (D) Immunoprecipitated SRK2E-GFP was analyzed with a nanoLC-MS/MS system. As examples, a non-phospho peptide of m/z = 691.8673 (blue) and a phospho-peptide of m/z = 970.8307 (red) are found in the structure of SRK2E and in extracted ion current (XIC) chromatograms. Arrows in XICs indicate the target peaks. Asterisks in red and black indicate probable phosphorylation sites detected in this study with the highest and less PTM scores, respectively. (E) An MS/MS spectrum derived from a phospho-peptide of m/z = 970.8307. Fragmented ions detected in this analysis are shown in red, and the top 15 ions were annotated in the spectrum. The results were confirmed through several replications.