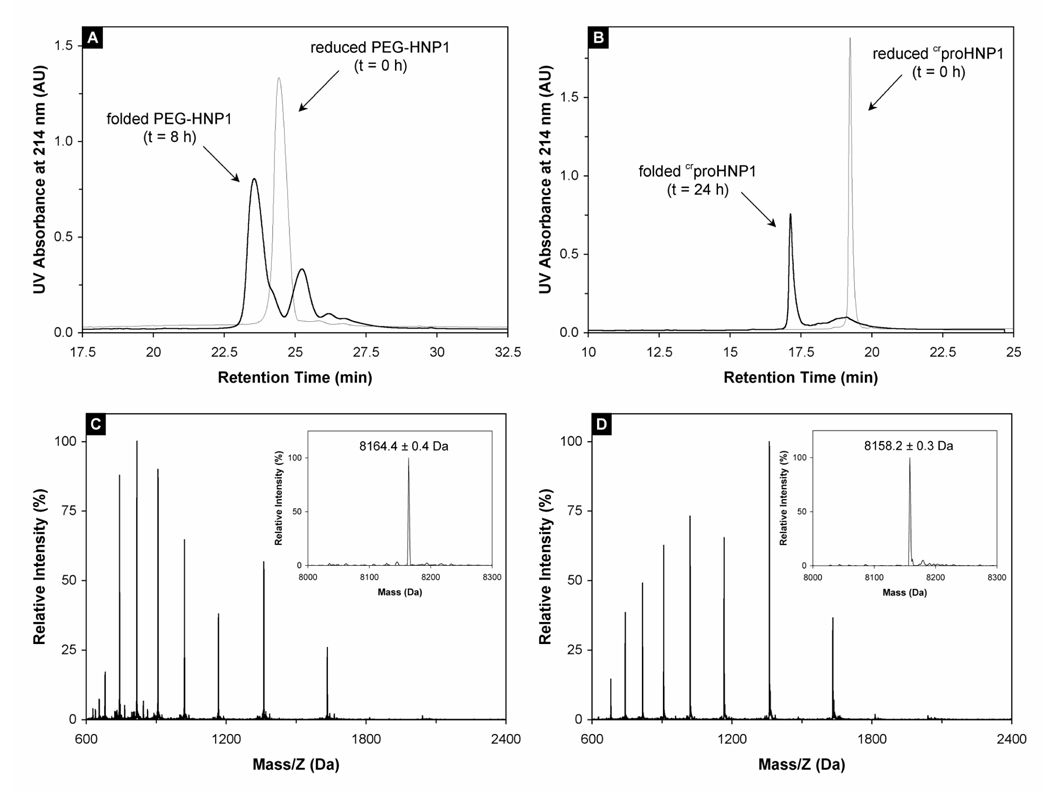
Figure 2.
(A) PEG-HNP1 before (thin line) and after (thick line) 8-h folding. RP-HPLC analysis was performed at 40 °C on a Waters Symmetry™ 300 C18 column (4.6 × 150 mm) using a linear gradient of 5–65% ACN containing 0.1% TFA at a flow rate of 1 ml/min over 30 min. 10 nmol peptide was injected. The 25.5 min peak in the 8 h trace was a major folding intermediate of PEG-HNP1. (B) crproHNP1 before (thin line) and after (thick line) 24-h folding. RP-HPLC analysis was carried out under identical chromatographic conditions. (C) Mass spectrometric analysis of unfolded crproHNP1. The measured molecular mass 8164.4 ± 0.4 Da is in agreement with the expected value of 8164.7 Da calculated based on the average isotopic compositions of reduced crproHNP1. (D) Folded crproHNP1 analyzed by ESI-MS. As expected, the folded defensin lost 6 mass units as a result of the formation of three disulfide bridges. Note that the characteristic shift in HPLC retention time and ESI charge envelope between reduced and oxidized crproHNP1 is consistent with the burial of hydrophobic residues associated with the folding of the molecule.